The equilibrium system of hydrogen, nitrogen, and ammonia can be written in several different ways: The equilibrium
Question:
The equilibrium system of hydrogen, nitrogen, and ammonia can be written in several different ways: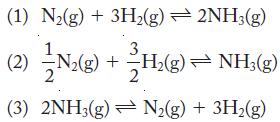
The equilibrium constant, Keq, for reaction 1 is 0.19 at 532 °C. Write the equilibrium constant expression and calculate Keq for reactions 2 and 3 at the same temperature.
Strategy
First, write the equilibrium constant expression for each equation, then examine the concentration terms to determine the relationship between equilibrium expressions. Use these relationships to calculate the relationships between equilibrium constants.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





