The Ostwald process is used to make nitric acid from ammonia. The first step of the process
Question:
The Ostwald process is used to make nitric acid from ammonia. The first step of the process is the oxidation of ammonia as pictured below: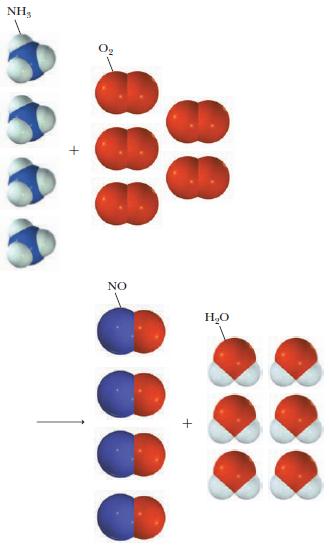
In an experiment, 50.0 g of each reactant is sealed in a container and heated so the reaction goes to completion.
(a) What is the limiting reactant?
(b) How much of the nonlimiting reactant remains after the reaction is completed? Assume that all of the limiting reactant is consumed.
Transcribed Image Text:
NH, NO HO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a O...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In the second step of the Ostwald process (see previous exercise), the nitrogen monoxide reacts with more O 2 to yield nitrogen dioxide. In an experiment, 75.0 g NO and 45.0 g O 2 are sealed in a...
-
The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen...
-
The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) 2NO(g) + O2(g) 2NO2(g) 3NO2(g) + H2O(l) ...
-
5. (8 points) (Determining a hidden "dot product vector") Consider the problem where one is given black-box access to a function f: {0, 1}" {0, 1} such that f(x) = a-z, where a {0,1}" is unknown....
-
Consider again the setup of Question 1. Suppose we want to price three European style call options written on one period (spot) Libor rates Li with i = 0, 1, 2, 3, as in the above case. Let these...
-
On July 1, Jerome received $200 in dividends from Melina Corporation on which it owns less than 20% of the voting stock. Complete the necessary journal entry selecting the account names from the...
-
How satisfied are you with your compensation (salary and benefits combined)? LO.1
-
The following data are taken from the general ledger and other records of Viejas Manufacturing Co. on January 31, the end of the first month of operations in the current fiscal year: Sales . . . . ....
-
Compute the expected return given these three economic states, their likelihoods, and the potential returns: (Round your answer to 2 decimal places.) Economic State Probability Return Fast growth...
-
In this discussion, we will explore one of the Neo-Freudian models of personality. Reflecting on your readings, respond to the following questions: Back in the 1950s, Erik Erikson argued that many...
-
E in is the energy being absorbed by an object, and E out is the energy being radiated. (a) If the temperature of an object is not changing, what does this tell us about E in and E out ? (b) If the...
-
When heated, potassium chlorate, KClO 3 , melts and decomposes to potassium chloride and diatomic oxygen. (a) What is the theoretical yield of O 2 from 3.75 g KClO 3 ? (b) If 1.05 g of O 2 is...
-
What is the manager trying to manage (i.e., optimize)?
-
Winston Electronics reported the following information at its annual meetings. The company had cash and marketable securities worth $1,235,740, accounts payables worth $4,160,391, inventory of...
-
Hooray Company has been manufacturing 12,000 units of Part A which is used to manufacture one of its products. At this level of production, the cost per unit is as follows: Direct materials P 4.80...
-
At the beginning of the period, the Grinding Department budgeted direct labor of $171,200 and property tax of $57,000 for 10,700 hours of production. The department actually completed 12,800 hours of...
-
The following information is available for Shamrock Corporation for the year ended December 31, 2025. Beginning cash balance $ 58,500 Accounts payable decrease 4,810 Depreciation expense 210,600...
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
Stetson Auto Inc. was incorporated on January 1, 2016 and commenced operations at that date. A $2,000 common share dividend was declared and paid on October 31, 2016. The following information was...
-
Find the reduced echelon form of each of the matrices given in Problems 120. c 1 26 + 4
-
Predict the products for each of the following: a. b. c. d. e. f. 1) O3 2) DMS 3) Excess LAH 4) H20 1) O3 2) DMS 3) Excess LAH 4) H20
-
Explain why the oscillations in the two-phase coexistence region using the RedlichKwong and van der Waals equations of state (see Figure 7.4) do not correspond to reality. Figure 7.4 140 120 100 304...
-
Propose a plausible mechanism for each of the following transformations. a. b. 1) MeMgBr 2) H20 1) Excess MeMgBr 2) H20
-
QUESTION 3 A business owns seven flats rented out to staff at R500 per month. All flats were tenanted Ist january 21 months rent was in arrears and as at 31st December 14 months' rent wa Identify the...
-
1. 2. 3. Select the Tables sheet, select cells A6:B10, and create range names using the Create from Selection button [Formulas tab, Defined Names group]. Select cells B1:F2 and click the Name box....
-
Tropical Rainwear issues 3,000 shares of its $18 par value preferred stock for cash at $20 per share. Record the issuance of the preferred shares. (If no entry is required for a particular...

Study smarter with the SolutionInn App


