The proton also has a spin quantum number, just like the electron. In an H atom, if
Question:
The proton also has a spin quantum number, just like the electron. In an H atom, if the spins of the p+ and e- are in the same direction, they are referred to as parallel. If they are in opposite directions, they are referred to as antiparallel. Suppose an H atom with its two particles having parallel spins is labeled Hp, and one with antiparallel spins is labeled Ha. Experiment shows that Hp is more stable than Ha. The energy between the two states has the same energy as a light wave with a wavelength of 21.0 cm.
(a) What is the difference in energy between the two states (in J)?
(b) What is the difference in energy between the two states (in J/mol)?
(c) The energy change of the reaction![]()
is 435.996 kJ/mol. What are the energy changes (in kJ/mol) of the following two reactions?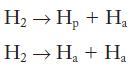
(d) Under the right conditions, the metal sodium can make an ionic compound with hydrogen, with the resulting formula NaH. What is the proper name of this compound?
(e) What is the oxidation number of Ha? Hp? Na and H in NaH?
(f) Are there two possible compounds NaHa and NaHp? Why or why not?
(g) Given that hydrogen is the most common element in the universe, explain why radioastronomers detect virtually no emissions from the sky of radiation that has the wavelength 21.0 cm.
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





