Question: Two compounds have the formula S 2 F 2 . Disulfur difluoride has the skeleton structure FSSF, whereas thiothionyl fluoride has the skeletal structure Determine
Two compounds have the formula S2F2. Disulfur difluoride has the skeleton structure F–S–S–F, whereas thiothionyl fluoride has the skeletal structure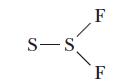
Determine Lewis structures for each compound.
S-S F F
Step by Step Solution
★★★★★
3.30 Rating (156 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

T S... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


