Use the data from the table of standard reduction potentials in Appendix H to calculate the standard
Question:
Use the data from the table of standard reduction potentials in Appendix H to calculate the standard potential of the cell based on each of the following reactions. In each case, state whether the reaction proceeds spontaneously as written or spontaneously in the reverse direction under standard-state conditions.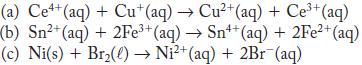
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Er...View the full answer

Answered By

Nicholas Maina
Throughout my tutoring journey, I've amassed a wealth of hands-on experience and honed a diverse set of skills that enable me to guide students towards mastering complex subjects. My proficiency as a tutor rests on several key pillars:
1. Subject Mastery:
With a comprehensive understanding of a wide range of subjects spanning mathematics, science, humanities, and more, I can adeptly explain intricate concepts and break them down into digestible chunks. My proficiency extends to offering real-world applications, ensuring students grasp the practical relevance of their studies.
2. Individualized Guidance:
Recognizing that every student learns differently, I tailor my approach to accommodate various learning styles and paces. Through personalized interactions, I identify a student's strengths and areas for improvement, allowing me to craft targeted lessons that foster a deeper understanding of the material.
3. Problem-Solving Facilitation:
I excel in guiding students through problem-solving processes and encouraging critical thinking and analytical skills. By walking learners through step-by-step solutions and addressing their questions in a coherent manner, I empower them to approach challenges with confidence.
4. Effective Communication:
My tutoring proficiency is founded on clear and concise communication. I have the ability to convey complex ideas in an accessible manner, fostering a strong student-tutor rapport that encourages open dialogue and fruitful discussions.
5. Adaptability and Patience:
Tutoring is a dynamic process, and I have cultivated adaptability and patience to cater to evolving learning needs. I remain patient through difficulties, adjusting my teaching methods as necessary to ensure that students overcome obstacles and achieve their goals.
6. Interactive Learning:
Interactive learning lies at the heart of my approach. By engaging students in discussions, brainstorming sessions, and interactive exercises, I foster a stimulating learning environment that encourages active participation and long-term retention.
7. Continuous Improvement:
My dedication to being an effective tutor is a journey of continuous improvement. I regularly seek feedback and stay updated on educational methodologies, integrating new insights to refine my tutoring techniques and provide an even more enriching learning experience.
In essence, my hands-on experience as a tutor equips me with the tools to facilitate comprehensive understanding, critical thinking, and academic success. I am committed to helping students realize their full potential and fostering a passion for lifelong learning.
4.90+
5+ Reviews
16+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the data from the table of standard reduction potentials in Appendix H to calculate the standard potential of the cell based on each of the following reactions. In each case, state whether the...
-
Use the standard reduction potentials in Appendix H to answer the following questions. (a) What products, if any, are formed when KClO 3 and KCl are mixed in an acid solution? (b) Which is a stronger...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
In September, Larson Inc. sold 45,000 units of its only product for $438,000, and incurred a total cost of $405,000, of which $43,000 was fixed costs. The flexible budget for September showed total...
-
The neighborhood cat likes to sleep on the roof of our shed in the backyard. The roofing surface is weathered galvanized sheet metal ( = 0.65, S = 0.8). Consider a cool spring day when the ambient...
-
The U.S. Navy requires that fighter pilots have heights between 62 in. and 78 in. a. Find the percentage of women meeting the height requirement. Are many women not qualified because they are too...
-
The Object Adapter pattern has great potential for design. Provide examples.
-
Polly, Molly, and Dolly are all single mothers. They each can earn $10 per hour working for up to 2,000 hours per year. Their government runs a welfare system that gives income benefits of $5,000 per...
-
1. List and explain what benefits Mimitos will obtain with the creation of a website and online sales platform.
-
The standard potential for the cell reaction is E = +0.460 V. Use the tabulated standard potential of the lead half-reaction to find the standard reduction potential of the uranium half-reaction.
-
For each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard...
-
Miller Co., which produces and sells skiing equipment, is financed as follows: Bonds payable, 10% (issued at face amount) $10,000,000 Preferred $1 stock, $10 par 10,000,000 Common stock, $25 par...
-
Dr. Kelly has a podiatry practice with total assets valued at $900,000. The liabilities of the business total $500,000. What is Dr. Kelly's podiatry practice's net worth?
-
Dr. Francis made the decision to buy two high end leather couches for the cancer center that he opened. He purchased the couches for a total of $7500. The couches will be used for 5 years and then...
-
balance in lilas account on March 1 was $18,200, and the balance on March 31 was $15,120. Costs incurred during the month were as follows: direct materials, $56,250; direct labor, $24,300; and...
-
Should auditors be paid by the client for audit work or by an independent agency! Why or why not?
-
Why can China be considered a revolutionary country in the payment game? reason.
-
The auditor is auditing accounts receivable. The company is a long-time client. Because of problems encountered in previous years, the auditor has assessed control risk as moderate and assigns a...
-
For the next several days, take notes on your listening performance during at least a half-dozen situations in class, during social activities, and at work, if applicable. Referring to the traits of...
-
Two mountain climbers are suspended by a rope as shown in Figure Q3.5. Which rope is most in danger of breaking? That is, which rope has the greatest tension? Figure Q3.5
-
A constant force of 400 N acts on a spacecraft of mass 8000 kg that has an initial velocity of 30 m/s. How far has the spacecraft traveled when it reaches a velocity of 5000 m/s?
-
You are riding in a car that starts from rest, accelerates for a short distance, and then moves with constant velocity. Explain why you feel a force from the back of your seat only while the car is...
-
Discuss the types of securities used currently in Pakistan and check marketable securities purchased by any company. Discuss 3 forms of markets also Discuss the performances of these markets in past...
-
1. Explain what is a bond and its characteristics, how does it differs from stocks? 2. Explain the main differences between bond and stocks. How would you choose to invest $1,000: bonds, stocks, or...
-
In 2018, Gig Harbor Heavy Metal, Inc. announced an after tax profit of $587,990. In 2019, it announced an after tax profit of $571,222. What is the percent change in after tax profits from 2018 to...

Study smarter with the SolutionInn App


