Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0
Question:
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 × 10-6 M iron(II) chloride is added to 20.0 mL of 3.0 × 10-4 M barium hydroxide.
Appendix F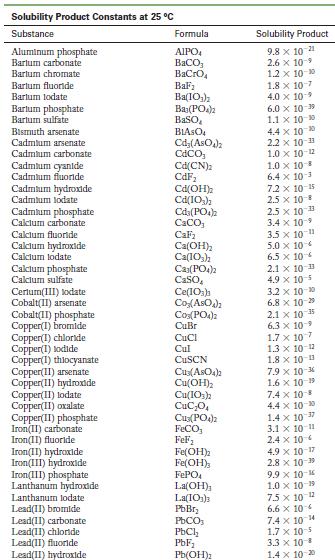

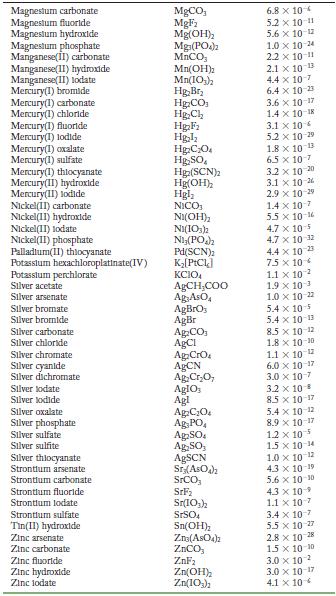
Transcribed Image Text:
Solubility Product Constants at 25 C Substance Aluminum phosphate Bartum carbonate Bartum chromate Barlum fluoride Barlum lodate Bartum phosphate Bartum sulfate Bismuth arsenate Cadmium arsenate Cadmium carbonate Cadmium cyanide Cadmium fluoride Cadmium hydroxide Cadmium lodate Cadmium phosphate Calcium carbonate Calcium fluoride Calcium hydroxide Calcium lodate Calcium phosphate Calcium sulfate Cerlum(III) lodate Cobalt(II) arsenate Cobalt(II) phosphate Copper(1) bromide Copper(1) chloride Copper(1) lodide Copper(1) thiocyanate Copper(11) arsenate Copper(II) hydroxide Copper(II) lodate Copper(II) oxalate Copper(II)phosphate Iron(II) carbonate Iron(11) fluoride Iron(II) hydroxide Iron(III) hydroxide Iron(III) phosphate Lanthanum hydroxide Lanthanum lodate Lead(11) bromide Lead(11) carbonate Lead(11) chloride Lead(11) fluoride Lead(II) hydroxide Formula AIPO BaCO3 BaCrO BaF Ba(10) Baz(PO4)2 BaSO BIASO Cd(AsO4)2 CdCO Cd(CN) CdF Cd(OH) Cd(10) Cd3(PO4)2 CaCO, CaF Ca(OH) Ca(10) C23(PO4)2 CaSO Ce(103)1 Co(ASO) CO3(PO4)2 CuBr CuCl Cul CuSCN Cuz(ASO4)2 Cu(OH) Cu(10)2 CuC0 Cu3(PO4)2 FeCO FeF Fe(OH)2 Fe(OH)3 FePO4 La(OH) La(IO3)3 PbBr PbCO PbCl PbF Pb(OH) Solubility Product 9.8 x 10 21 2.6 x 10-9 1.2 x 10-10 1.8 x 10-7 4.0 x 109 6.0 x 10-19 1.1 x 10-10 4.4 x 100 2.2 x 10-11 1.0 x 10-12 1.0 x 10-8 6.4 x 10- 7.2 x 10-15 2.5 x 10-8 2.5 x 10-3 3.4 x 10 3.5 x 10 11 5.0 x 10 6.5 x 10 2.1 x 10 11 4.9 x 10-5 3.2 x 10-20 6.8 x 10-29 10-1 2.1 x 6.3 10- 1.7 x 107 1.3 x 10-12 1.8 x 10-13 7.9 x 10-6 1.6 x 10 19 7.4 x 10-8 4.4 x 10-0 1.4 x 10-7 3.1 x 10-11 2.4 x 10 4.9 x 10-17 2.8 x 10-9 9.9 x 10- 1.0 x 10-19 75 x 10-2 6.6 x 10 7.4 x 10 4 1.7 x 10-5 3.3 x 10 B 1.4 x 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Q 53 10 ...View the full answer

Answered By

Amit Singh
When I was preparing for IIT-JEE exam which is one of the toughest exam of India , I use to teach mathematics and physical chemistry to my friend.
During college days I use to teach structural analysis, strength of materials,RCC i.e. Civil Engineering topics to my friends.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10 mL 0.0010 M AgNO 3 is added to 10 mL 0.0010 M Na 2 SO 4 . Appendix F Solubility Product...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0 mL of 1.0 10 -6 M magnesium chloride is added to 80.0 mL of 1.0 10 -6 M potassium fluoride....
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0 mL of 0.010 M NaOH is added to 75.0 mL of a 0.10 M solution of magnesium chloride? Appendix F...
-
How are Bit coins different from VCU1, VCU2, and VCU3 currencies? How are they similar? What is the primary economic threat of Bit coins?
-
Give an example of how, under absorption costing, operating income could fall even though the unit sales level rises.
-
The Bureau of Transportation Statistics reports on-time performance for airlines at major U.S. airports. JetBlue, United, and US Airways share terminal C at Bostons Logan Air-port. The percentages of...
-
A testing laboratory requires a new DRIE machine with a cost of $\$ 499,000$, and in this high-tech industry the machine will have a salvage value of $\$ 40,000$ at the end of 10 years of service....
-
Martinez Manufacturing Inc. showed the following costs for last month: Direct materials ....... $7,000 Direct labor ......... 3,000 Manufacturing overhead ... 2,000 Selling expense ........ 8,000...
-
1. What is the output of the following code? Explain. class Super { public void show() { System.out.println("Base::show() called"); class Sub extends Super { public void show() {...
-
Calculate the solubility of copper(II) iodate, Cu(IO 3 ) 2 (K sp = 7.4 10 -8 ), in (a) Water. (b) A 0.10 M copper(II) nitrate solution.
-
Calculate the solubility of barium sulfate (K sp 1.1 10 -10 ) in (a) Water. (b) A 0.10 M barium chloride solution.
-
The management of the Diners Delight franchised restaurant chain is in the process of establishing quality- control charts for the time that its service people give to each customer. Management...
-
On October 1, 2019, Jarvis Co. made a credit sale to a foreign customer for 100,000 LCU (a foreign currency). The payment is to be collected on Feb. 1, 2020. A forward exchange contract was acquired...
-
Bloomfield Beverages Ltd has adopted the practice of financing its balance sheet through the use of promissory notes. Bloomfield just issued a new 180-day note, with a face value of $50 million. The...
-
Aaliyah Brown owns a small bakery named Sweet Delights and is considering expanding her product line with different dessert options. She's presented with the following alternatives: Dessert Good...
-
Complete this question by entering your answers in the tabs below. Required 1Required 2 Prepare a classified balance sheet at its December 31 year-end. (Hint: remember to include accumulated...
-
In process costing, factory rent and utilities are debited to the ________. Question content area bottom Part 1 A. Finished Goods Inventory account B. Manufacturing Overhead account C. WorkinProcess...
-
Donna Donie, CFA, has a client who believes the common stock price of TRT Materials (currently $58 per share) could move substantially in either direction in reaction to an expected court decision...
-
A copper sphere of 10-mm diameter, initially at a prescribed elevated temperature T;, is quenched in a saturated (1 atm) water bath. Using the lumped capacitance method, estimate the time for the...
-
An inclined manometer similar to the one shown in Figure 3.14 is used for sensitive pressure measurement. It is inclined at an angle of 25 degrees above the horizontal and uses red gage fluid with a...
-
A meteorologist reports a high pressure system with barometric pressure of 790 mm of mercury and then later in the year a low pressure system with a pressure of 738 mm of mercury. What is the total...
-
What is the pressure, in psig, at the bottom of a swimming pool that is 10 ft deep?
-
We assume that the stock price S is governed under the martingale measure by the Black-Scholes stochastic differential equation dS = St (r dt + dWt), So > 0, where > 0 is the stock price volatility...
-
Taylor Simmons owns an online store that sells small appliance parts. She wishes to see the percentage of sales revenue earned less than a particular amount for various parts sold in her shop. What...
-
Analyze the risks of a Mezzanine financing in a growth company like the "Elephant Bar Restaurant" . Also, evaluate the deal structure of this financing in order to manage the investment risk of the...

Study smarter with the SolutionInn App


