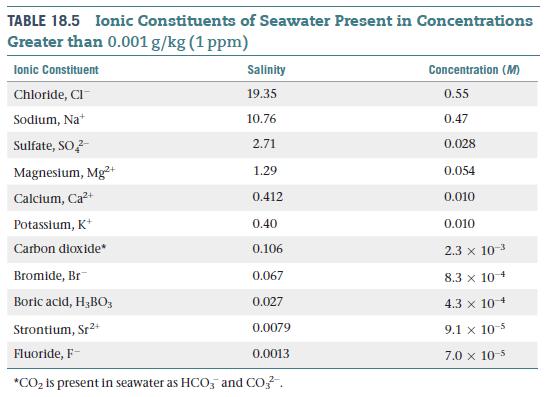
Although there are many ions in seawater, the overall charges of the dissolved cations and anions must
Question:
Although there are many ions in seawater, the overall charges of the dissolved cations and anions must maintain charge neutrality. Consider only the six most abundant ions in seawater, as listed in Table 18.5 (Cl−, Na+, SO42−, Mg2+, Ca2+, and K+), calculate the total charge in Coulombs of the cations in 1.0 L of seawater. Calculate the total charge in Coulombs of the anions in 1.0 L of seawater. To how many significant figures are the two numbers equal?
Table 18.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





