Aspirin has the structural formula At body temperature (37C), K a for aspirin equals 3 10
Question:
Aspirin has the structural formula
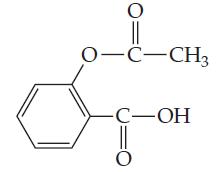
At body temperature (37°C), Ka for aspirin equals 3 × 10-5. If two aspirin tablets, each having a mass of 325 mg, are dissolved in a full stomach whose volume is 1 L and whose pH is 2, what percent of the aspirin is in the form of neutral molecules?
Transcribed Image Text:
0-C-CH3 -С—ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
Body temperature 37 The percent of ionization is The percent of aspirin in neutra...View the full answer

Answered By

Desmond Boakye-Agyemang
I have been teaching and preparing students to write exams for more than seven years and with hands on practicals of chemistry through higher education, teaching has been very easy and students appreciate my efforts and way of teaching.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Related Video
For this experiment, we\'ve compared the freshness of flowers by keeping them in three separate bottles filled with water, aspiring water, and food plant
Students also viewed these Sciences questions
-
When perspiration on the human body absorbs heat, some of the perspiration turns into water vapor. The latent heat of vaporization at body temperature (37 C) is 2.42 106 J/kg. The heat absorbed is...
-
(a) Calculate the percent ionization of a 0.20 M solution of the monoprotic acetylsalicylic acid (aspirin) for which Ka 5 3.0 3 10-4. (b) The pH of gastric juice in the stomach of a certain...
-
One suggested treatment for a person who has suffered a stroke is immersion in an ice-water bath at 0C to lower the body temperature, which prevents damage to the brain. In one set of tests, patients...
-
discusses how a reseller can service both a consumer and an industrial market from the same store location. Provide an example of a retailer and detail the differences in their marketing activities.
-
Refer to the cause-and-effect diagram on page 17. The workers have now noticed that a delay could occur: (a) On the fourth floor at the pharmacy (b) On the third floor at the practitioners' station...
-
Mrs. Hanrahans precalculus class collected data on the length (in centimeters) of a pendulum and the time (in seconds) the pendulum took to complete one back-andforth swing (called its period). The...
-
Check whether the following can define probability distributions and explain your answers. (a) \(f(x)=\frac{1}{4}\) for \(x=10,11,12,13\) (b) \(f(x)=\frac{2 x}{5}\) for \(x=0,1,2,3,4,5\) (c)...
-
On September 18, 2014, Gerald received land and a building from Frank as a gift. Frank's adjusted basis and the fair market value at the date of the gift are as follows: No gift tax was paid on the...
-
a. Prepare a horizontal analysis of the income statements. Round percentages to one decimal place. Amazon.com, Inc. Income Statements For the Years Ended December 31 (in millions) Revenues: Product...
-
A rock band's tour bus, mass M, is accelerating away from a stop sign at rate a when a piece of heavy metal, mass M/5, falls onto the top of the bus and remains there. a. Show that the bus's...
-
A sample of 7.5 L of NH 3 gas at 22C and 735 torr is bubbled into a 0.50-L solution of 0.40 M HCl. Assuming that all the NH 3 dissolves and that the volume of the solution remains 0.50 L, calculate...
-
What is the pH at 25 C of water saturated with CO 2 at a partial pressure of 1.10 atm? The Henry's law constant for CO 2 at 25 C is 3.1 10 2 mol/L-atm. The CO 2 is an acidic oxide, reading with H 2...
-
Test for exactness. If exact, solve. If not, use an integrating factor as given or obtained by inspection or by the theorems in the text. Also, if an initial condition is given, find the...
-
Discuss how the characteristics of bounded rationality, indi-vidual profit maximization, and opportunistic behavior lead to different forms of governance in transactions between two or more actors.
-
Explain the different options of aligning and harmonizing cost accounting systems across supply chain partners. Under which conditions will each option be an appropriate choice for the firms involved?
-
Discuss the applicability of target costing in supply chains and describe possible application scenarios.
-
Name and describe the most important prerequisites for successful IOCM implementation. Which factors make IOCM success more likely?
-
Name and describe different options of exchanging cost-relevant information between supply chain partners.
-
Mona viewed herself as a creative individual who had chosen to go to law school for economic reasons. Mona's undergraduate majors were creative writing and American Indian studies. Mona was very...
-
Should we separate the debt and equity features of convertible debt? Team 1: Pro separation: Present arguments in favor of separating the debt and equity features of convertible debt. Team 2: Against...
-
Vector A starts at point (1, - 1, - 3) and ends at point (2, - 1, 0). Find a unit vector in the direction of A.
-
Given vectors A = x2 y3 z, B = x2 y z3, and C = x4 y2 z2, show that C is perpendicular to both A and B.
-
In Cartesian coordinates, the three corners of a triangle are P1 (0, 4, 4). P2 (4, - 4, 4), and P3 (2, 2, - 4) find the area of the triangle.
-
QUESTION #2 : The age of the internet as well as the ensuing rise of social networking in the form of FaceBook, Instagram, Twitter and TikTok has significantly changed the way individuals process and...
-
Question 5 In 50 words or more, explain how advertising is implemented through each type of media listed below. Advertising Media Types How Advertising is Implemented Through Each Type of Media i....
-
1. Research and outline the Lean Method and how it applies to marketing a products. Explain in one fifty words. 2. Outline your understanding of market research and what is should include: Be...

Study smarter with the SolutionInn App


