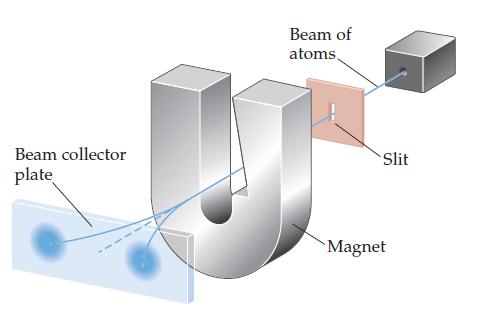
In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field.
Question:
In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams.
(a) What is the significance of the observation that the single beam splits into two beams?
(b) What do you think would happen if the strength of the magnet were increased?
(c) What do you think would happen if the beam of hydrogen atoms were replaced with a beam of helium atoms? Why?
(d) The relevant experiment was first performed by Otto Stern and Walter Gerlach in 1921. They used a beam of Ag atoms in the experiment. By considering the electron configuration of a silver atom, explain why the single beam splits into two beams.
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





