Naturally found uranium consists of 99.274% 238 U, 0.720% 235 U, and 0.006% 233 U. As we
Question:
Naturally found uranium consists of 99.274% 238U, 0.720% 235U, and 0.006% 233U. As we have seen, 235U is the isotope that can undergo a nuclear chain reaction. Most of the 235U used in the first atomic bomb was obtained by gaseous diffusion of uranium hexafluoride, UF6(g).
(a) What is the mass of UF6 in a 30.0-L vessel of UF6 at a pressure of 695 torr at 350 K?
(b) What is the mass of 235U in the sample described in part (a)?
(c) Now suppose that the UF6 is diffused through a porous barrier and that the change in the ratio of 238U and 235U in the diffused gas can be described by Equation 10.23. What is the mass of 235U in a sample of the diffused gas analogous to that in part (a)?
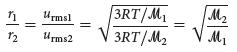
(d) After one more cycle of gaseous diffusion, what is the percentage of 235UF6 in the sample?
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





