Question: Potassium and hydrogen react to form the ionic compound potassium hydride. (a) Write a balanced equation for this reaction. (b) Use data in Figures 7.10
Potassium and hydrogen react to form the ionic compound potassium hydride.
(a) Write a balanced equation for this reaction.
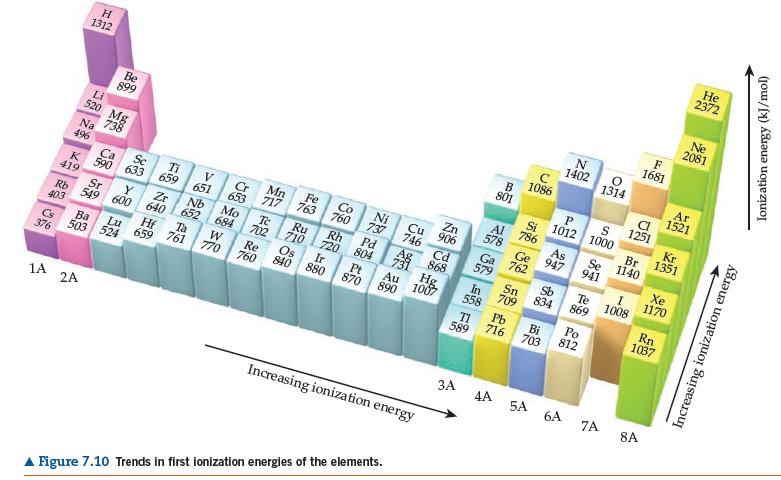
(b) Use data in Figures 7.10 and 7.12 to determine the energy change in kJ/mol for the following two reactions:
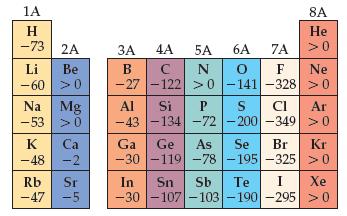
Figures 7.12
K(g) + H(g) → K+(g) + H-(g)
K(g) + H(g) → K-(g) + H+(g)
(c) Based on your calculated energy changes in (b), which of these reactions is energetically more favorable (or less unfavorable)?
(d) Is your answer to (c) consistent with the description of potassium hydride as containing hydride ions?
2372 Ne 2081 H F 1681 1312 1402 1314 Be 899 Ar 1521 1086 B 801 Li S 1251 520 Mg 738 Si 1012 | 1000 Kr 1351 Na 496 Br 1140 786 Zn 906 Al 578 As 947 Se 941 Fe Co Mn 717 Ni 737 Ti Cu 746 Sc Cr 653 Ge 762 Ca 763 760 Ga Xe 1170 K 590 633 659 651 Cd 579 419 Sr Pd 804 Pt 870 Rh 720 Te 710 Os 840 880 Ru Ag Sb Te 869 Nb 652 Ta 761 Mo 684 868 Hg 1007 Y Zr 1008 Sn 709 640 702 834 Rb 403 In 558 549 600 Rn 1037 Ir Au W 770 Re 760 890 Pb 716 Po 812 Lu Bi 703 659 Cs 376 TI 589 503 524 1A 2A 4A 5A 6A 7A 8A Increasing ionization energy A Figure 7.10 Trends in first ionization energies of the elements. Increasing ionization energy Ionization energy (kJ/mol)
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it
a When potassium is heated in the current of hydrogen gas potassium hydride is formed The balanced c... View full answer

Get step-by-step solutions from verified subject matter experts


