Sodium oxide (Na 2 O) adopts a cubic structure with Na atoms represented by green spheres and
Question:
Sodium oxide (Na2O) adopts a cubic structure with Na atoms represented by green spheres and O atoms by red spheres.
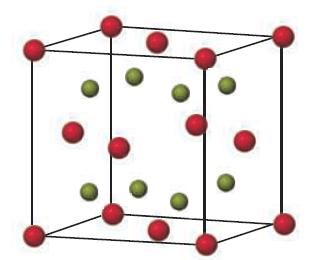
(a) How many atoms of each type are there in the unit cell?
(b) Determine the coordination number and describe the shape of the coordination environment for the sodium ion.
(c) The unit cell edge length is 5.550 Å. Determine the density of Na2O.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





