The AsAs bond length in elemental arsenic is 2.48 . The ClCl bond length in Cl 2
Question:
The As—As bond length in elemental arsenic is 2.48 Å. The Cl—Cl bond length in Cl2 is 1.99 Å.
(a) Based on these data, what is the predicted As—Cl bond length in arsenic trichloride, AsCl3, in which each of the three Cl atoms is bonded to the As atom?
(b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?
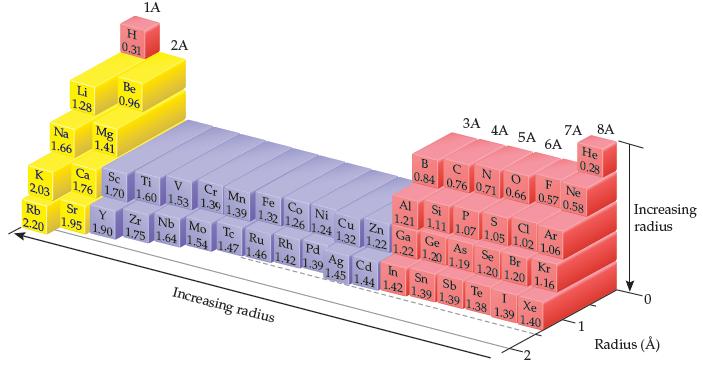
Transcribed Image Text:
1A 2A 0.31 8A 7A 3A AA 5A 6A Be 0.96 He 0,28 Li 128 N 10.84 0.76 0.71 0.66 0.57 0.58 B Increasing radius Ne Mg Na 1.66 1,41 Al Si P. Ar Cr Mn 1.21 1.11 1.07 1.05 1.02 1.06 V. Fe Co Ni Cu Zn Ga Ca Se Ti K. 170 1.60 1.53 1.3 1.391.32 1.26 1.24 1.32 1.22 1.22 1.20 1.19 1.201.20 1.16 Ge As Se Br Kr 2,03 Nb Mo 1.95 190 1.75 1.64 1.54 1.47 1.46 |142 1.39 Sr Y Zr Te Ru Rh Pd Rb Ag Cd In Sn Sb Te 2.20 1.45 1.44 1421391.39 |1.38 1.39 1.40 Xe Radius (Å) Increasing radius
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a The As x As bond length in elemental arsenic is 24...View the full answer

Answered By

SRIRAM GUPTA
RIGHT FROM THE BEGINNING OF MY STUDIES, I LOVED TO KNOW ABOUT CHEMISTRY. I WAS INTERESTED IN HOW CHEMICALS WORK AND WHAT THEIR PROPERTIES ARE. I CHOOSE CHEMISTRY AS MY HONOR SUBJECT IN GRADUATION AND I READ ABOUT THE STRUCTURE OF CHEMICALS, THEIR PROPERTIES AND HOW THEY WORK.
I TEACH PHYSICS AND CHEMISTRY TO INTERMEDIATE STUDENTS IN KRISHNA CLASSES COACHING. I PREPARE KIDS IN SUCH A WAY THAT THEY NOT ONLY TOP IN THEIR HOME EXAM BUT THEY ALSO PERFORM WELL IN BIG COMPETITIONS IN THE COUNTRY.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
Estimate the As I bond length from the data in Figure 7.6, and compare your value to the experimental As I bond length in arsenic triiodide, AsI 3 , 2.55 . 1A H 2A 7A 8A 3A AA 5A 6A 0.37 0.32...
-
The Alaska Pipeline carries 2 million barrels per day of crude oil from Prudhoe Bay to Valdez covering a distance of 800 miles. The pipe diameter is 48 in. and it is insulated with 4 in. of...
-
A crystalline silicon wafer of 10 cm diameter and 1.0 mm thickness is coated with a thin film of elemental arsenic metal (As), which is a semiconductor dopant. The As-coated silicon wafer is baked in...
-
Explain fully the role played by unplanned investment in inventories in determining equilibrium in the Keynesian model. Use the examples of AD > GDP and AD < GDP to illustrate your answer.
-
The shape of y = x2, but reflected across the x-axis and shifted right 3 units and up 4 units Write an equation for a function that has a graph with the given characteristics.
-
How do environmental factors, such as climate change and habitat fragmentation, influence the rate and pattern of speciation across different taxa ? Explain
-
What is a contra-asset? Give an example of one.
-
The following transactions of Great Value Pharmacies occurred during 2018 and 2019: 2018 Mar. 1 Borrowed $390,000 from Bartow Bank. The six-year, 13% note requires payments due annually, on March 1....
-
SportsWorld purchased equipment costing $10,000. The equipment has a residual value of $1,000, and an estimated useful life of 5 years or 36,000 shoes. Actual units produced during the year were...
-
A Global private bank is aggressively looking to leverage technology to improve customer experience and reduce operational costs. Over the last few years, it has tied up with at least five startups...
-
In Table 7.8, the bonding atomic radius of neon is listed as 0.58 , whereas that for xenon is listed as 1.40 . A classmate of yours states that the value for Xe is more realistic than the one for Ne....
-
The following observations are made about two hypothetical elements A and B: The AA and BB bond lengths in elemental A and B are 2.36 and 1.94 , respectively. A and B react to form the binary...
-
Explain how an objective state of mind and due care contribute to a PAs qualifications to conduct a financial statement audit.
-
I am standing with a baseball in my hand. The mass of the baseball is 1 4 5 g ( 0 . 1 4 5 kg ) . The ball is held 1 . 2 m from the ground. What is the potential energy of the baseball in my hand...
-
A golf ball is hit with horizontal velocity of 1 4 m / s and vertical velocity of 1 0 m / s . a. How long ( how many seconds ) is the ball moving upward before coming back down? b. How long does...
-
Theorem: The negative of every irrational number is irrational. Proof: 1. Suppose there is some irrational number p such that -p is rational. 2. -p = m/n, where m and n are both integers and n # 0 3....
-
Hypertext is: Very large text Structured text in which transitions can be made on selected marks Text typed on a computer Text that uses a large font
-
A branch in an algorithm can be a good marker to indicate _ _ _ _ _ _ _ _ _ _ when developing a program. a . ) a point to gather input b . ) a point to produce output c . ) a potential pattern d . )...
-
StraightArrow Banking & Investments competes with EndRun Financial Services in the Tri-Cities area. Currently, StraightArrow is featuring its StraightDeal investment fund. Open StraightDeal.pdf and...
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
The methane molecule, CH4, has the geometry shown in Figure 2.19. Imagine a hypothetical process in which the methane molecule is "expanded," by simultaneously extending all four C-H bonds to...
-
World energy supplies are often measured in the unit of quadrillion British thermal units (1012 Btu), generally called a "quad." In 2015, world energy consumption is projected to be 5.81 1017 kJ....
-
Consider the conversion of compound A into compound B: A -- B. For both compounds A and B, Hj > 0. (a) Sketch an enthalpy diagram for the reaction that is analogous to Figure 5.23. (b) Suppose the...
-
Identify an area of Financial Services where you feel significant opportunities lie in relation to process management. Why do you feel this?
-
1. Given the functions f(x) = x + 1 and g(x) = 3-x, determine an equation for the combined function y = f(x)+ g(x). 2. If f= {(-7, 1), (-5, 8), (3, 11), (5,-1)) and g = {(-6, 4), (-5, 3), (-1, 7),...
-
What is urban economics and what components of a city usually results in higher demand of a city's real estate. Describe at least three

Study smarter with the SolutionInn App


