(a) What is the average kinetic energy of a gas molecule at 20.0C (room temperature)? (b) Find...
Question:
(a) What is the average kinetic energy of a gas molecule at 20.0°C (room temperature)?
(b) Find the rms speed of a nitrogen molecule (N2) at this temperature.
Strategy for (a)
The known in the equation for the average kinetic energy is the temperature.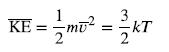
Before substituting values into this equation, we must convert the given temperature to kelvins. This conversion gives T=(20.0+ 273) K = 293 K.
Strategy for (b)
Finding the rms speed of a nitrogen molecule involves a straightforward calculation using the equation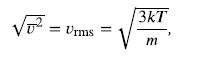
but we must first find the mass of a nitrogen molecule. Using the molecular mass of nitrogen N2 from the periodic
table,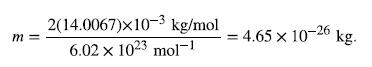
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





