The color of dyes results from the preferential absorption of certain wavelengths of light. Certain dye molecules
Question:
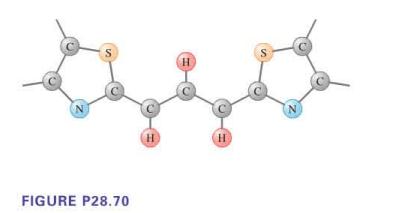
The color of dyes results from the preferential absorption of certain wavelengths of light. Certain dye molecules consist of symmetric pairs of rings joined at the center by a chain of car- bon atoms, as shown in Figure P28.70. Electrons of the bonds along the chain of carbon atoms are shared among the atoms in the chain, but are repelled by the nitrogen-containing rings at the end of the chain. These electrons are thus free to move along the chain but not beyond its ends. They look very much like a particle in a one-dimensional box. For the molecule shown, the effective length of the "box" is 0.85 nm. Assuming that the electrons start in the lowest energy state, what are the three longest wavelengths this molecule will absorb?
Step by Step Answer:

College Physics A Strategic Approach
ISBN: 9780321595492
2nd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field





