An atom of Na can combine with an atom of Cl to form the molecule NaCl. It
Question:
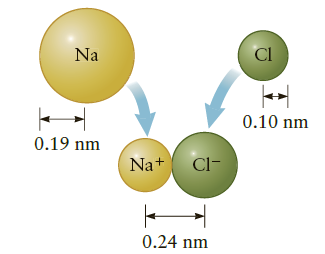
An atom of Na can combine with an atom of Cl to form the molecule NaCl. It is a good approximation to view this process as two steps (Fig. P18.87) in which the Na atom gives up an electron to the Cl atom, forming a positive ion (Na+) and a negative ion (Cl-). These ions are then attracted by the electric force, forming the stable molecule Na+Cl-. To calculate the changes in electric potential energy associated with these processes, assume the outermost electron in Na is r = 0.19 nm from the nucleus (this is the atomic radius of Na) and the separation of the ions in Na+Cl- is 0.24 nm.
(a) What is the energy required to remove an electron and create Na+?
(b) The energy gained by adding an electron to Cl to make Cl- is called the electron affinity and has been measured by chemists to be 349 kJ/mol of Cl atoms. Express this value in eV/atom.
(c) What is the electric potential energy of Na+Cl-?
(d) Combine your answers from parts (a) through (c) to estimate the energy gained by making Na+Cl-. Give some reasons why your answer differs from the measured dissociation energy of this molecule, 4.26 eV. In particular, what aspect of your calculation in part (a) causes it to overestimate the ionization energy of Na?
Figure P18.87
Step by Step Answer:

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano





