Oxygen, O 2 , dissolves quite well in a class of compounds known as liquid perfluorocarbons?so well
Question:
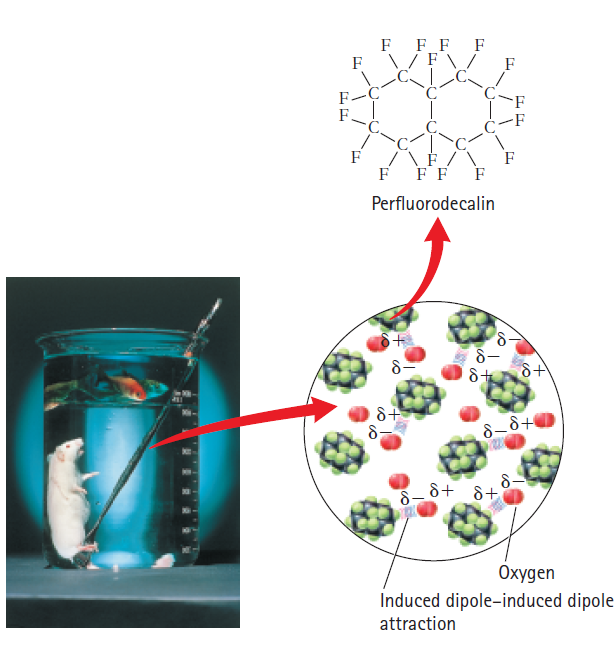
Oxygen, O2, dissolves quite well in a class of compounds known as liquid perfluorocarbons?so well that oxygenated perfluorocarbons can be inhaled in a liquid phase, as is demonstrated by the rodent shown below the water-bound goldfish. Do you suppose perfluorocarbon molecules are polar or nonpolar? Why would the rodent drown if it were brought up to the water layer and the goldfish die if they swam down into the perfluorocarbon layer? How might perfluorocarbons be used to clean our lungs or serve as artificial blood? When is it okay to sacrifice the lives of animals for scientific research?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:




