In terms of Le Chateliers Principle, explain why steam reforming is done at low pressures while methanol
Question:
In terms of Le Chatelier’s Principle, explain why steam reforming is done at low pressures while methanol synthesis is performed at moderate to high pressures. Explain why reforming is done at high temperature and methanol synthesis is performed at low to moderate temperatures.
Perform an analysis of the converter loop by determining the composition and flow rate of the purge stream using a basis of 100 kmol of feed from the MUG compressor.
a. Assume that the feed has a composition that is 3 mole% methane, 8% CO2, 15% CO, and the remainder hydrogen.
b. Revise the feed composition to that calculated in Problem 13.12. As given in the Process Description, the molar flow rate of material to the MSR is 7.8 times the flow rate of fresh feed.
c. To simplify calculations, assume that the liquid leaving the flash drum contains no methane, CO1, CO, or hydrogen and that the gas contains no water or methanol. The single-pass conversions of CO and CO2 are 15% and 10%, respectively. You may be assisted in your calculations by assuming flow rates of components in the recycle stream mixed with fresh synthesis gas entering the converter loop. In this approach the recycle stream is known as a tear stream, and an iterative solution will be required to determine the requested values. Such calculations are easily performed using a simulation program of the type described in Chapter 10, or you may write your own program or spreadsheet to obtain the desired results. If you develop a spreadsheet to perform the calculations, direct substitution of calculated values of component flow rates in the tear stream for new estimates may suffice. (This is the method of successive substitution described in Appendix A.2.)
Problem 13.12
As covered in the Process Description, there are three primary reactions that occur in the MSR. These are given by Equations 13.3, 13.4, and 13.5. However, determination of chemical equilibrium among the species H2, CO, CO2, H2O, and CH3OH involves only two of the three reactions because each reaction is a linear combination of the other two. Cherednichenko gives an approximation for the equilibrium relationships in Equation 13.3 (see Problem 13.13) and in Equation 13.5:
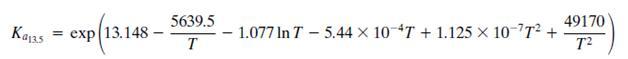
The equilibrium Ka13.5 constant is defined by the relationship
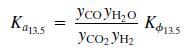
Where T is in kelvin and Kϕ13.5 accounts for nonideal behavior of the gas phase.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





