Question: Use a commercial flowchart simulation program such as HYSYS or ASPEN to simulate the ammonium nitrate manufacturing process described in Example 10.3-3. Example 10.3-3 FINES
Use a commercial flowchart simulation program such as HYSYS or ASPEN to simulate the ammonium nitrate manufacturing process described in Example 10.3-3.
Example 10.3-3
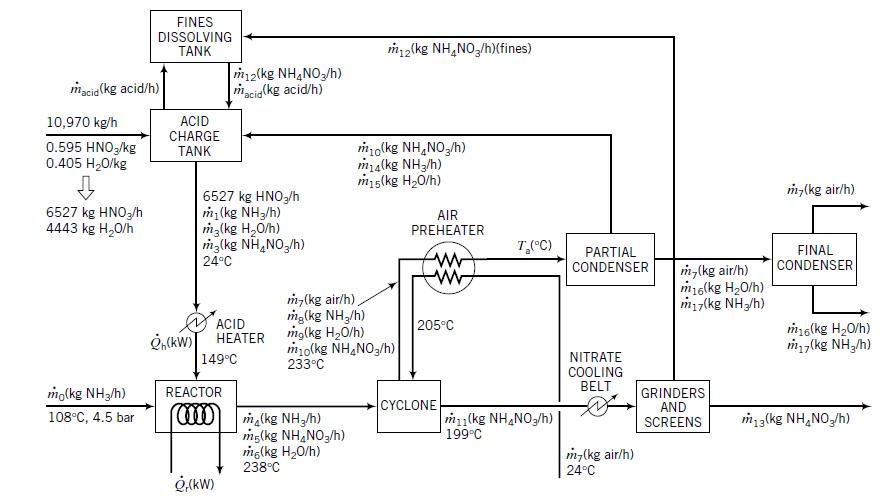
FINES DISSOLVING TANK m2(kg NH,NO,/h)(fines) m12(kg NH,NO,/h) macig(kg acid/h) macia (kg acid/h) 10,970 kg/h ACID CHARGE 0.595 HNO,/kg 0.405 H20/kg mo(kg NH,NO,/h) ma(kg NH3/h) m15(kg H20/h) TANK m,(kg air/h) 6527 kg HNO3/h 4443 kg H20/h 6527 kg HNO,/h m (kg NH3/h) m3(kg H,0/h) m3(kg NH,NO,/h) AIR PREHEATER T,(C) PARTIAL CONDENSER FINAL CONDENSER 24C m,(kg air/h) m16(kg H20/h) miy(kg NH/h) m,(kg air/h), mg(kg NH,/h) mg(kg H20/h) m10(kg NH,NO3/h) 233C ACID TER 205C m6(kg H20/h) m (kg NH,/h) NITRATE COOLING BELT 149C mo(kg NH3/h) REACTOR GRINDERS AND SCREENS CYCLONE m1(kg NH,NO3/h)| 199C 108C, 4.5 bar ma(kg NH,/h) mg(kg NH,NO,/h) mg(kg H20/h) 238C m3(kg NH,NO,/h) m-(kg air/h) 24C
Step by Step Solution
3.37 Rating (169 Votes )
There are 3 Steps involved in it
HX1 S1 S7 HX2 S2 COOL1 M3 COOL2 S3 M4 M2 ... View full answer

Get step-by-step solutions from verified subject matter experts


