Iron (Fe) is an essential trace metal that is needed by all cells. It is required, for
Question:
Iron (Fe) is an essential trace metal that is needed by all cells. It is required, for example, for synthesis of the heme groups and iron sulfur centers that are part of the active site of many proteins involved in electron-transfer reactions; it is required in hemoglobin, the main protein in red blood cells. Iron is taken up by cells by receptor mediated endocytosis. The iron-uptake system has two components: a soluble protein called transferrin, which circulates in the bloodstream; and a transferrin receptor a transmembrane protein that, like the LDL receptor in Figure 15−33, is continually endocytosed and recycled to the plasma membrane. Fe ions bind to transferrin at neutral pH but not at acidic pH. Transferrin binds to the transferrin receptor at neutral pH only when it has an Fe ion bound, but it binds to the receptor at acidic pH even in the absence of bound iron. From these properties, describe how iron is taken up, and discuss the advantages of this elaborate scheme.
Figure 15−33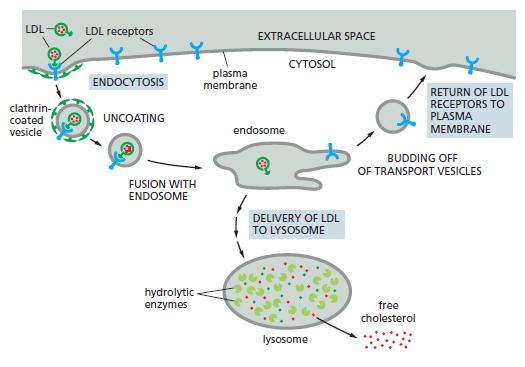
Step by Step Answer:

Essential Cell Biology
ISBN: 9780393680362
5th Edition
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter





