When 1-methoxy-2-methylpropene is treated with HCl, the major product is 1-chloro-1-methoxy-2-methylpropane. Although this reaction proceeds via an
Question:
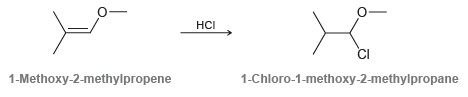
Transcribed Image Text:
HCI 1-Methoxy-2-methylpropene 1-Chloro-1-methoxy-2-methylpropane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 86% (15 reviews)
In this case the lesssub...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When 1-methylcyclohexene is treated with HCl, a Markovnikov addition is observed. How would you use 1 H NMR spectroscopy to determine that the major product is indeed the Markovnikov product?
-
When the (R, R) isomer of the amine shown is treated with an excess of methyl iodide, then silver oxide, then heated, the major product is the Hofmann product. (a) Draw the structure of the major...
-
When 2-methyl-2-propanol is treated with sulfuric acid, 2-methylpropene is formed. Show all of the steps in the mechanism for this reaction. Don't forget to use curved arrows to show the movement of...
-
Arrange the following events in the correct temporal sequence during eukaryotic cell division, starting with the earliest: (a) condensation of the chromosomes, (b) Movement of chromosomes to the...
-
What led to the eventual collapse of Enron under Lay and Skilling? How did the top leadership at Enron undermine the foundation values of the Enron Code of Ethics?
-
Big Rock Brewery Inc., which is based in Calgary, Alberta, is a publicly listed company. The companys products are sold across the country in nine provinces and three territories as well as being...
-
According to Eq. 6.134, the \(x\)-velocity in fully developed laminar flow between parallel plates is given by \[ u=\frac{1}{2 \mu}\left(\frac{\partial p}{\partial x} ight)\left(y^{2}-h^{2} ight) \]...
-
Preparing a classified balance sheet Required Use the following information to prepare a classified balance sheet for Little Co. at the end of 2010. Accounts receivable ...... $42,500 Accounts...
-
Describe a situation situation where physical agents pose a potential hazard? What agents are present? How do these agents pose hazards? What steps have been taken to control the physical agents and...
-
Whole Foods Markets financial statements from the chapter. The average common shareholders equity for 2012 was $3.397 billion, and a 40% income tax rate should be used as needed. Required: Whole...
-
Which of the following make a person less likely to have health insurance? Select one or more answers from the choices shown. a. Working for a larger firm. b. Being a low-wage worker. c. Being...
-
A patient named Jen visits Dr. Jan. Dr. Jan is nearly certain that Jen only has a cold. But because Dr. Jan is afraid of malpractice lawsuits, she orders an extensive battery of tests just to make...
-
What are some areas that require estimates to record transactions under the matching rule?
-
In spray drying if the particle size is reduced from 120 microns to 100 microns, what will be the increase in heat transfer rate?
-
Research some of the AI services available from Amazon. Make a list of five to ten of those you find most interesting, describe what they do and why these seem appealing. Investigate industries...
-
If the heat transfer coefficient of the hot and the cold fluid sides are neglected, what will be the change in the overall heat transfer coefficient if aluminum is used instead of stainless steel?
-
Research cloud computing outages among major cloud service providers (AWS, Microsoft Azure, Google). Have you found any cloud computing outages that have taken place in the last three years? What...
-
If a copper cylinder of length 5 cm and diameter of 2 cm is heated from one end at 100C, while the other end is at 30C, what is the rate of heat transfer. If the material is made of iron what will be...
-
(a) Find the vertical asymptotes of the function (b) Confirm your answer to part (a) by graphing the function. ? + 1 22 2.x3
-
Consider the function f and its graph. a. Estimate the zeros of the area function b. Estimate the points (if any) at which A has a local maximum or minimum. c. Sketch a graph of A, for 0 x 10,...
-
Is a nucleus that absorbs at 6.50 more shielded or less shielded than a nucleus that absorbs at 3.20 ? Does the nucleus that absorbs at 6.50 require a stronger applied field or a weaker applied...
-
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, ordiastereotopic: (a) (b) C (c)
-
How many types of nonequivalent protons are present in each of the followingmolecules? (c) (b) CH3CH2CH20CH3 (a) H3C CH3 Naphthalene (e) (d) C=CH2 CO2CH2CH3 Ethyl acrylate Styrene
-
1) Explain how a Preschool program can help to bring a sense of belonging for the child? 2) It is important for a program to provide physical environments that are age-appropriate and that support...
-
Your company decided to organize a special dinner to appreciate all the employees and it has been proposed that the dinner to be held in the middle of December 2021. You have been appointed as the...
-
Select a two-dimensional image to analyze that is either a work of art (photograph, illustration, or painting) or an advertisement. What is the FTC analysis?Use the FTC palette to break down the...

Study smarter with the SolutionInn App


