Question: (a) Verify Equation 36.8 by considering a single-electron atom with nuclear charge Ze instead of e.(b) Calculate the ionization energies for single-electron versions of helium,
(a) Verify Equation 36.8 by considering a single-electron atom with nuclear charge Ze instead of e.(b) Calculate the ionization energies for single-electron versions of helium, oxygen, lead, and uranium.?
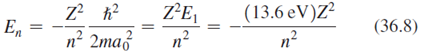
ZE, n? (13.6 eV)Z n? (36.8) En n2 2ma
Step by Step Solution
3.52 Rating (172 Votes )
There are 3 Steps involved in it
a Equation 368 is the formula for the ionization energy of an electron in an atom given by ... View full answer

Get step-by-step solutions from verified subject matter experts


