Question: Lithium fluoride, LiF, has the same crystal structure as NaCl and therefore has essentially the same Madelung constant a. Its ionic cohesive energy is -10.5
Lithium fluoride, LiF, has the same crystal structure as NaCl and therefore has essentially the same Madelung constant a. Its ionic cohesive energy is -10.5 eV and the value of n in Equation 37.4 is 6.25 Find equilibrium ionic separation LiF.?
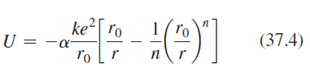
ke ro U = (37.4) o [r
Step by Step Solution
★★★★★
3.46 Rating (166 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The equilibrium ionic separation in LiF can be determined using the Madelun... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


