Question: Solid oxide fuel cells use unique electrolytesa hard ceramic, based on a solid state solution of zirconium oxide stabilized by the element yttrium, and which
Solid oxide fuel cells use unique electrolytes—a hard ceramic, based on a solid state solution of zirconium oxide stabilized by the element yttrium, and which will conduct oxygen ions when hot enough (which is about 1,000°C). The anode is a Ni/ceramic material and the cathode is an exotic material such as lanthanum strontium manganite. It works similarly to a PEM fuel cell. Air is applied to the cathode and hydrogen to the anode. It is about 60% efficient and, if it has an application, it will be to sophisticated stationary power plant systems. Write down the anode and cathodic reactions; what is the theoretical voltage of such a cell?
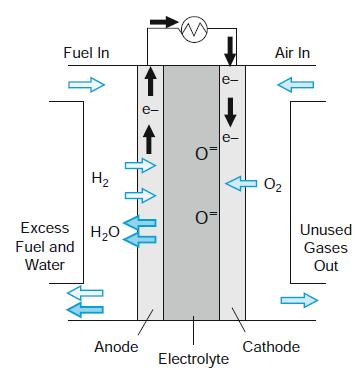
Fuel In Air In e- e- e- H2 O2 Excess H20 Unused Fuel and Gases Water Out Anode Cathode Electrolyte
Step by Step Solution
3.39 Rating (161 Votes )
There are 3 Steps involved in it
The anode reaction in a solid oxide fuel cell is the oxidation of hydrogen gas ... View full answer

Get step-by-step solutions from verified subject matter experts


