The equilibrium constant for the conjugate acid-base pair is 8.00 x 10 -5 . From the additional
Question:
The equilibrium constant for the conjugate acid-base pair
![]()
is 8.00 x 10-5. From the additional information
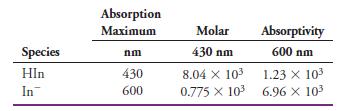
(a) Calculate the absorbance at 430 nm and 600 nm for the following indicator concentrations: 3.00 x 10-4 M, 2.00 x 10-4 M, 1.00 x 10-4 M, 0.500 x 10-4 M, and 0.250 x 10-4 M.
(b) Plot absorbance as a function of indicator concentration.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





