In a substudy, participants will be subdivided into two equal groups according to the severity of previous
Question:
Ophthalmology
A study is being planned to assess whether a topical antiallergic eye drop is effective in preventing the signs and symptoms of allergic conjunctivitis. In a pilot study, at an initial visit, participants are given an allergen challenge; that is, they are subjected to a substance that provokes allergy signs (e.g., cat dander) and their redness score is noted 10 minutes after the allergen challenge (visit 1 score). At a follow-up visit, the same procedure is followed, except that participants are given an active eye drop in one eye and the placebo in the fellow eye 3 hours before the challenge; a visit 2 score is obtained 10 minutes after the challenge. The data collected are shown in Table 8.26.
Table 8.26: Effect of an eye drop in reducing ocular redness among participants subjected to an allergen challenge
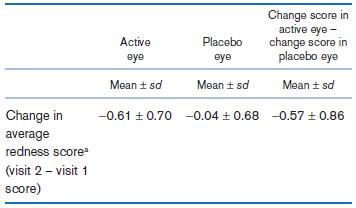
aThe redness score ranges from 0 to 4 in increments of 0.5, where 0 is no redness at all and 4 is severe redness.
Step by Step Answer:






