a) Show that the variation of the C P with pressure at constant temperature is given by
Question:
a) Show that the variation of the CP with pressure at constant temperature is given by
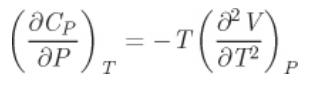
b) Use this result to show that the CP and CV in the ideal-gas state are independent of pressure.
c) If the expansion coefficient β of a liquid is assumed to be approximately independent of pressure, what do you conclude for the relationship between CP and pressure in the compressed liquid state?
Transcribed Image Text:
() =-(+) T P
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a To demonstrate how the specific heat variation at constant pressure The value of C P pressure at c...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:
Students also viewed these Engineering questions
-
Show that the variation of atmospheric pressure with altitude is given by P = P0eay, where a = pog/P0, P0 is atmospheric pressure at some reference level y = 0, and p0 is the atmospheric density at...
-
The Natural Gas Consumption Case a. Use the explained variation and the unexplained variation as given on the computer output to calculate (within rounding) the F(model) statistic. b. Utilize the...
-
The Fresh Detergent Case a. Use the explained variation and the unexplained variation as given on the computer output to calculate (within rounding) the F( model) statistic. b. Utilize the F(model)...
-
1. Two football fans are listening to the Grey Cup game on the radio, one in Montreal, where the game is being played, the other in Vancouver, 3692 km away. How much sooner does the Montreal fan hear...
-
The Moller Skycar M400 is a flying car known as a personal air vehicle (PAV) that is expected to be FAA-certified by December 31, 2011. The cost is $985,000, and a $100,000 deposit will hold one of...
-
Occupational prestige is a statistic developed by sociologists to measure the status of one's occupation. Occupational prestige is also a component of what sociologists call socioeconomic status, a...
-
Ron Chambers arrives at work early on Friday morning. His anxiety has been growing throughout his final week of training with Mid-Town Office Products. Today Ron is going to work with his sales...
-
1. Based on the information in this case, provide examples, for Siemens, of at least four strategically required organizational outcomes, and four required workforce competencies and behaviors. 2....
-
A clothing company sells ski jackets every winter but must decide in the summer how many jackets to produce. Each jacket costs $65 to produce and ship and sells for $129 at retail stores. For the...
-
A Carnot cycle operating between 600 C and 25 C absorbs 1000 kJ of heat from the high-temperature reservoir. The work produced is used to power another Carnot cycle which transfers 1000 kJ of heat...
-
A young engineer notices in her plant that 1-kg blocks of brick are routinely removed from a 800 C oven and are let stand to cool in air at 25 C. Conscious about cost-cutting and efficiency, she...
-
What does 'planning and control' mean to air traffic controllers?
-
Over the past 15 years, Volkswagen Group (VW) acquired several fiefdoms- Audi, Lamborghini, Bentley, Bugatti, Skoda, SEAT - that jealously guarded their brand and continuously rebelled against...
-
Companies can shift physical resources and capital relatively easily among national markets where labor is cheaper. But laborers generally face greater difficulty moving to other countries for higher...
-
Using the benefits you have identified, construct a presentation for share holders, explaining why corporate resources were being invested in this way,
-
Imagine that the nations of the world suddenly cut off all trade with one another and the people in each nation were able to consume only products their own nation produced. What products previously...
-
Since 1978, according to the World Bank, Chinas GDP has grown at an annual rate of 9 percent. The high and sustained growth has been based on investment, low-cost manufacturing, and global exports....
-
What are the pros and cons of life insurance comparison indexes?
-
Whats the difference between an ordinary annuity and an annuity due? What type of annuity is shown below? How would you change the time line to show the other type of annuity?
-
Use the van der Waals EOS to estimate the fugacity of propane at each of the following conditions. A. T = 200 K, P = 0.5 bar (vapor) B. T = 300 K, P = 1 bar (vapor) C. T = 400 K, P = 5 bar (gas) D. T...
-
Estimate the boiling points of toluene at pressures of P = 0.1, 0.5, 1, and 5 bar: A. The Antoine equation B. The Clausius-Clapeyron equation, with H vap = 33.2 kJ/mol at the normal boiling point of...
-
Estimate the boiling points of n-hexane at pressures of P = 0.1, 0.5, 1, and 5 bar, using the following methods. A. The Antoine equation B. The Clausius-Clapeyron equation, with H vap = 28.85 kJ/mol...
-
Excel Toys, a manufacturer of plastic toys for children, requires constant supply of high-density polyethylene (HDPE) in the resin form. HDPE is available in the resin form and one tonne costs...
-
You need to mix 42 kilograms of mortar. Each kilogram of mortar mix requires 0.03 liters of water. how many liters of water do you need? (Round your answer to the nearest tenth of a liter )
-
2. (4 Points) Assume a consumer's preferences and the market can be defined as follows: U-2X+3Y, P=0.50, P =1, and M=20. y Graph the consumer optimization problem in (X, Y) space. Clearly label the...

Study smarter with the SolutionInn App


