Escape velocity from Earth's atmosphere is (10.8 mathrm{~km} / mathrm{s}). a. Based on that number, to what
Question:
Escape velocity from Earth's atmosphere is \(10.8 \mathrm{~km} / \mathrm{s}\).
a. Based on that number, to what temperature would \(\mathrm{He}, \mathrm{O}_{2}, \mathrm{CO}_{2}\) need to be heated so that their mean velocity would allow them to escape into interplanetary space?
b. Given the Maxwell-Boltzmann velocity distribution of equation (3.1), an escape velocity from the moon of \(2.4 \mathrm{~km} / \mathrm{s}\), and a mean moon surface temperature of \(127^{\circ} \mathrm{C}\), what fraction of \(\mathrm{O}_{2}\) would have enough speed to escape the moon's gravity? Why might the moon have no atmosphere?
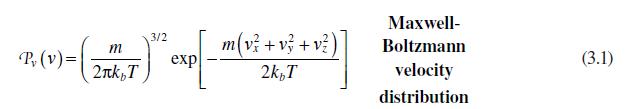
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





