Five moles of gas are confined in a piston-cylinder device (Figure 6-5). At the beginning of the
Question:
Five moles of gas are confined in a piston-cylinder device (Figure 6-5). At the beginning of the process, the gas has T = 300 K and V = 100 L. If the gas is compressed isothermally to a final volume of 15 L, how much work is required, and how much heat is added or removed? Assume the heat capacity is constant at CV = 30 J/mol · K and that the gas is modeled by the van der Waals equation of state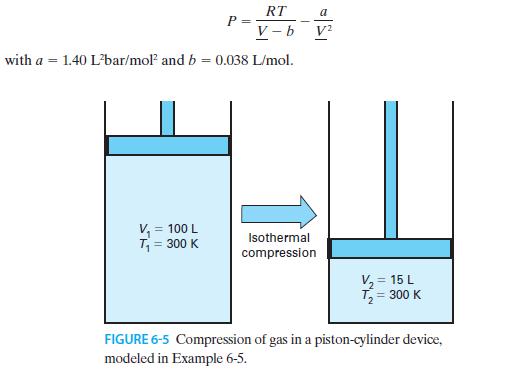
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





