For an ethylene glycol n-butyl ether (1) + water (2) system at 310 K with 70% by
Question:
For an ethylene glycol n-butyl ether (1) + water (2) system at 310 K with 70% by mass water, determine if the system is one stable liquid phase or two stable liquid phases at equilibrium. If the latter, provide the mass fraction of the co-existing phases.
figure 13-3
Transcribed Image Text:
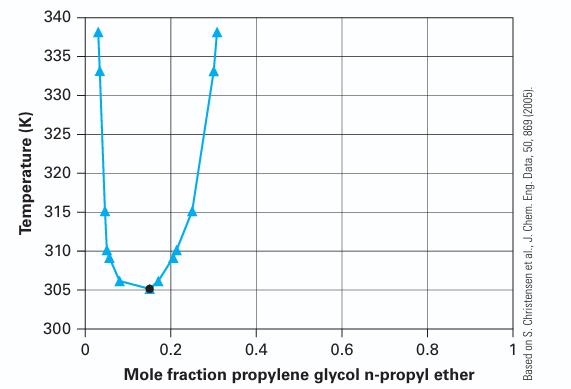
Temperature (K) 340 335 330 325 320 315 310 305 300 0 0.2 0.4 0.6 0.8 Mole fraction propylene glycol n-propyl ether Based on S. Christensen et al., J. Chem. Eng. Data, 50, 869 (2005).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Figure 1.28 shows a circuit with five elements. If P1 = 205 W, P2 = 60W, P4 = 45W, P5 = 30W, calculate the power p3 received or delivered by element 3. 3
-
Select all that is /are true about the financial markets. A. Financial markets bring together the buyers and sellers of debt and equity together . B. Stocks trading on an organized exchange such as...
-
Amortization of Bond Premium or Discount The appropriate method of amortizing a premium or discount on issuance of bonds is the effective interest method. Required: 1. What is the effective interest...
-
Use the Ratio Test to determine if each series converges absolutely or diverges. 8 n=1 nt (-4)"
-
Why is feedback a necessary component of the communication process?
-
ND Paint Designs began operations on April 1, 2021. The company completed the following transactions in its first month: Instructions a. For each transaction, indicate: (1) the basic type of account...
-
A 10-m-long, 5.042-cm I.D. copper pipe has two fully open gate valves, a swing check valve, and a sudden enlargement to a 9.919-cm I.D. copper pipe. The \(9.919 \mathrm{~cm}\) copper pipe is \(5.0...
-
On December 31, 2016, Vail Company owned the following assets: Vail computes depreciation and amortization expense to the nearest whole year. During 2017, Vail engaged in the following transactions:...
-
QwikShare is a new not-for-profit organization that will rent low-emissions automobiles at QwikStops in suburban areas in order to provide an environmentally friendly transportation option to its...
-
This problem expands upon Example 15-4. A reaction vessel is rigid and has a volume of 500 L and initially contains 10 moles of o-xylene. The liquid phase is exposed to catalyst that facilitates...
-
30 mol/s of hydrogen gas and 15 mol/s of air, each compressed to 25 bar, enter a steady state reactor as shown in Figure 15-5, where the nitrogen in the air reacts with the hydrogen to form ammonia:...
-
Let W. Z V be complementary subspaces in a vector space V, as in Exercise 2.2.24. (a) Prove that if (w1,...,wj} form a basis for W and {z1.......zk} a basis for Z, then {w1,..., wj, z1,.... zk} form...
-
research all about the following: 1) LINUX SYSTEM 2) LINUX COMPONENTS 3) LINUX DISTRIBUTIONS
-
Find a photograph of yourself that also shows examples of goods, services, and factors of production. Then respond to the questions. Identify three to five goods or services in the photograph, and...
-
What is the difference between linux/x64/shell/bind_tcp and linux/x64/shell_bind_tcp ? What is the difference between linux/x64/shell/bind_tcp and linux/x64/shell/reverse_tcp ? What is the...
-
The criminal justice field inherently revolves around physicality, stress and ability to take action when necessary. These attributes make the need for a structured, focused approach to sustained...
-
Given that the power P developed by a wind turbine is a function of the diameter D and rotational speed N of the rotor, and of the density p, dynamic viscosity and velocity V of the airstream,...
-
Entrance to a prestigious MBA program in India is determined by a national test where only the top 10% of the examinees are admitted to the program. Suppose it is known that the scores on this test...
-
The registrar of a college with a population of N = 4,000 full-time students is asked by the president to conduct a survey to measure satisfaction with the quality of life on campus. The following...
-
The following data are available for a gas: To calculate the molar volume at 25 C, 12 bar, our team came up with two different suggestions: (a) Linear interpolation for Vbetween the given pressure, ...
-
Determine the temperature and phase of water from the following information. If the phase is a vapor-liquid mixture, report the fraction of vapor and liquid. a) The specific volume of water is 100 cm...
-
In 1656, Otto von Guericke of Magdeburg presented his invention, a vacuum pump, through a demonstration that became a popular sensation. A metal sphere made of two hemispheres (now known as the...
-
Cranshaw Business Services (CBS) operates an information technology (IT) consulting firm out of two offices: Detroit and Los Angeles. Corporate services, such as legal, finance, and personnel, are...
-
Regular Company produces audio equipment, specifically headphones and speakers. A new CEO has just been hired and announces a new policy that if a product cannot earn a markup of at least 25 percent,...
-
1. The band is breaking up and Rob, Sue, Tim and Vito each want the tour bus. Using the method of sealed bids, Rob bids $4200, Sue bids $5200, Tim bids $5400, and Vito bids $7300 for the bus. Since...

Study smarter with the SolutionInn App


