For the methanol (1) + acetone (2) system at 101.325 kPa, what is the K-factor for each
Question:
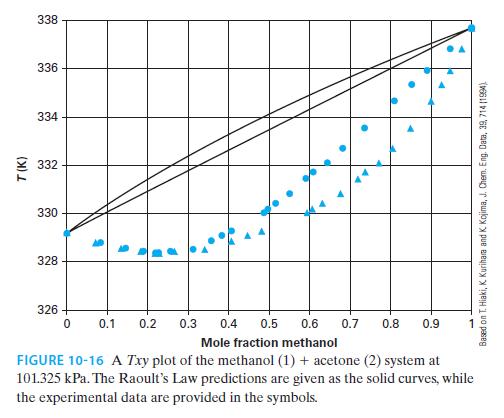
For the methanol (1) + acetone (2) system at 101.325 kPa, what is the K-factor for each substance at 332 K? What is the relative volatility at 332 K? Use Figure 10-16.
Transcribed Image Text:
T(K) 338 336 334 332 330 328 326 0 S 1 1 ● 0.1 0.2 0.3 0.4 0.5 4 0.7 · 4 0.6 Mole fraction methanol FIGURE 10-16 A Txy plot of the methanol (1) + acetone (2) system at 101.325 kPa. The Raoult's Law predictions are given as the solid curves, while the experimental data are provided in the symbols. 0.8 0.9 Based on T. Hiaki, K. Kurihara and K. Kojima, J. Chem. Eng. Data, 39, 714 (1994).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

Ajay Negi
Hi, I've completed my degree in engineering (Information Technology) from an NIT. Currently working as a software engineer. Wish to impart quality education to the future generation.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
In Exercises use the graph of ' to sketch a graph of and the graph of ". 20 16 12 8 4 -8-4 +x + 4 8 12 16
-
Which type(s) of power did Elon Musk have: reward power, coercive power, legitimate power, or expert power? Considering the creation of Tesla and SpaceX (manufacturing), is Twitter the right business...
-
Determine the force in each member of the space truss in E9.3.27 if the magnitudes of F and F are 8 kip and 4 kip, respectively. State whether each member is in tension or compression. 2 ft F2 2 ft...
-
Apply the product rule for exponents, if possible. y 4 y 5 y 6
-
In what kinds of organizations might affective commitment be especially important?
-
Based on information from MRINetwork, some job applicants are required to have several interviews before a decision is made. The number of required interviews and the corresponding probabilities are:...
-
The wings of old airplanes are often strengthened by the use of wires that provided cross-bracing as shown in Fig. P9.106. If the drag coefficient for the wings was 0.020 (based on the planform...
-
A machine is purchased at the beginning of 2011 for $42,000. Its estimated life is eight years. Freight costs on the machine are $3,000. Installation costs are $1,600. The machine is estimated to...
-
Unit Replacement Product ABCDE A Quantity 1,000 Unit Cost $ 14 Cost $ 16 Forest Company has five products in its inventory. Information about ending inventory follows. Unit Selling Price $ 20 B 800...
-
Calculate the Raoults Law predicted vapor-phase composition and bubble-point pressure for an equimolar mixture of methanol (1) + ethanol (2) at 273.15 K. Please report the pressure in bar.
-
Find the efficiency of each of the following Carnot heat engines. A . T H = 500 K, T C = 300 K B . T H = 500C, T C = 300C C . T H = 1000F, T C = 500F D . T H = 1000R, T C = 500R
-
Prepare your net worth statement using the guidelines presented in Exhibit 14-3.
-
11. Comparing Cash Flows- What would be more valuable, receiving $600 today or receiving $750 in three years if interest rates are 8 %? 12.Future Value - Compute the future value in year 11 of a...
-
Write a JAVA program that reads a list of 25 values from the user. Put the values in an array. The program should read the array and then calculate and display the average of the even input values...
-
If you use a volumetric pipet to transfer 25 mL of a 0.501 M stock solution into a 100 mL volumetric flask and then filled the flask to the calibration mark with deionized water, what would the...
-
Syarikat Jenin using process costing to gather information on production costs. All direct materials are added at the beginning of the process and conversion costs are evenly incurred throughout the...
-
1.What are the top skills that one should look for in a consultant? 2. Should we be aiming for a specialist or more of a generalist type of individuals? What are the main differences? 3. How is the...
-
More and more households are struggling to pay utility bills given a shaky economy and high heating costs (The Wall Street Journal, February 14, 2008). Particularly hard hit are households with homes...
-
What are some of the various ways to implement an awareness program?
-
Imagine you are tasked with designing a single dishwasher for both the European and American markets. Determine a set of global, social, environmental, and economic issues you would have to consider...
-
Find a product specification sheet for a consumer product such as an automobile, appliance, TV, motor, or something similar, and determine whether the specifications are easy to interpret. For...
-
Develop 15 ways to determine which direction is north. Provide descriptions and/or sketches of each idea.
-
Schedule of Cost of Goods Manufactured Sydney Company reported the following amounts for October: Beginning raw materials inventory $144,000 Beginning work-in-process inventory 252,000 Beginning...
-
Cullumber Company started business on January 1, 2024. Some of the events that occurred in its first year of operations follow: Transactions 1. Equipment that cost $208,400 was purchased on February...
-
Determine the amount that would be reported in ending merchandise inventory on October 15 using the FIFO inventory costing method. Enter the transactions in chronological order, calculating new...

Study smarter with the SolutionInn App


