In the production of superconducting wire tube, the alloy is reacted with oxygen to produce the appropriate
Question:
In the production of superconducting wire tube, the alloy is reacted with oxygen to produce the appropriate ceramic phase. Oxygen at a concentration \(c_{o}\) flows through the inner tube and diffuses into the metal, undergoing a chemical reaction to produce the ceramic as shown in Figure P5.31. The reaction rate has been measured as a function of position and follows:
\[-r_{a}(\text { moles } / s)=k^{\prime \prime} c_{o}\left(\frac{r_{o}-r}{r_{o}-r_{i}}\right)^{2}\]
Pure nitrogen flows on the outside of the tube to scavenge away any oxygen that doesn't react by the time it reaches the surface.
a. Draw the control volume for deriving the mass balance for the system.
b. Using the general balance equation, derive the differential mass balance giving the concentration profile of oxygen in the superconductor.
c. What are the boundary conditions for this problem?
d. Solve the differential equation for the concentration profile.
e. What is the flux of \(a\) at \(r=r_{i}\) ?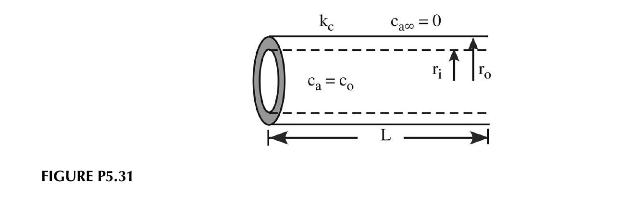
Step by Step Answer:





