N-formylmorpholine can be used as a solvent in an extraction process for producing high-purity aromatic compounds. To
Question:
N-formylmorpholine can be used as a solvent in an extraction process for producing high-purity aromatic compounds. To that end, liquid–liquid equilibrium data has been prepared for this compound with a variety of aromatics, including methylcyclopentane. Using the LLE diagram in Figure E13-1 for the methylcyclopentane (1) + N-formylmorpholine (2) system, answer the following questions
A. For an equimolar mixture at 320 K, what is the composition of the stable phase(s)?
B. For an equimolar mixture at 420 K, what is the composition of the stable phase(s)?
C. Estimate the UCST for this system and the com position of the UCST. D. Provide the structure for both compounds. By examining the structure, explain why this system would produce a miscibility gap
Figure E13-1
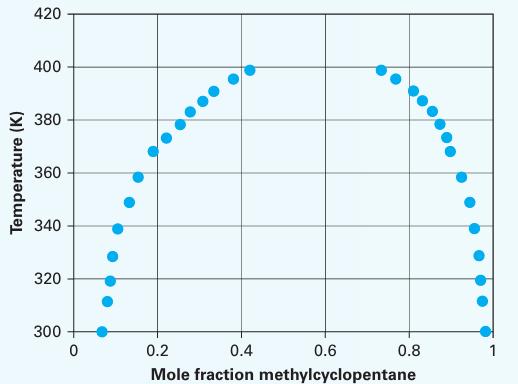
Figure 13-5
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





