The compound chloromethane can be synthesized by the chemical reaction: This reaction progresses by a free-radical mechanism
Question:
The compound chloromethane can be synthesized by the chemical reaction:
![]()
This reaction progresses by a free-radical mechanism and typically requires high temperatures.
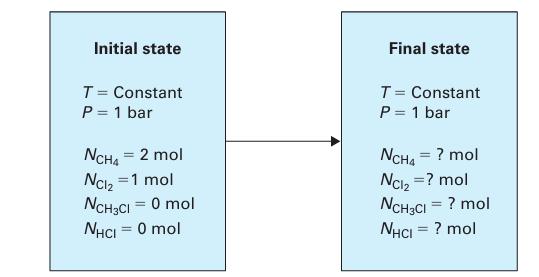
The reaction is carried out in a closed vessel (Figure 14-7) at P = 1 bar and constant temperature. The reactor initially contains 2 moles of methane and 1 mole of chlorine. Assuming this is the only chemical reaction that occurs, determine the extent of reaction at equilibrium for temperatures of:
A. 298.15 K
B. 800 K
Figure 14-7.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





