The system is 1 kg of nitrogen, initially at T = 300 K and P = 4
Question:
The system is 1 kg of nitrogen, initially at T = 300 K and P = 4 bar. For each of the following cases, find the change in entropy of the system using Figure 2-3, then find the change in entropy again by assuming nitrogen is an ideal gas and using the data in Appendix D.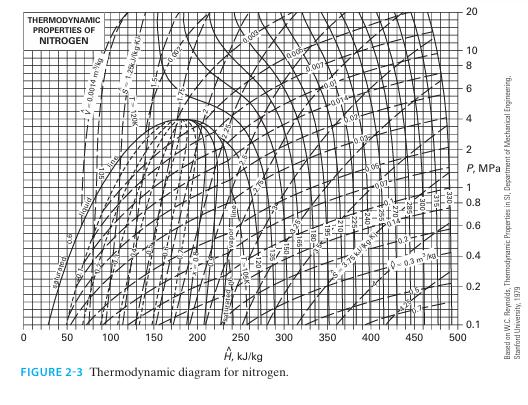 A. The nitrogen is isobarically heated to 330 K.
A. The nitrogen is isobarically heated to 330 K.
B. The nitrogen is isobarically cooled to 225 K.
C. The nitrogen is isothermally compressed to P = 10 bar.
D. The nitrogen is compressed to T = 330 K and P = 10 bar.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





