This problem examines the generalization mentioned in Section 7-3, that the Lee-Kesler generalized approach should not be
Question:
This problem examines the generalization mentioned in Section 7-3, that the Lee-Kesler generalized approach should not be applied to highly polar compounds.
A. Choose three temperatures and three pressures that, for water, fall within the range 0.7 r r
B. For each of the nine data points identified in part A, estimate the molar volume of water from the Lee-Kesler approach, and compare to the data in the steam tables.
C. Repeat parts A and B for methane. Use the same reduced temperatures and pressures that you identified in part A for water, and compare the results of the Lee-Kesler approach to the data in Figure 7-1.
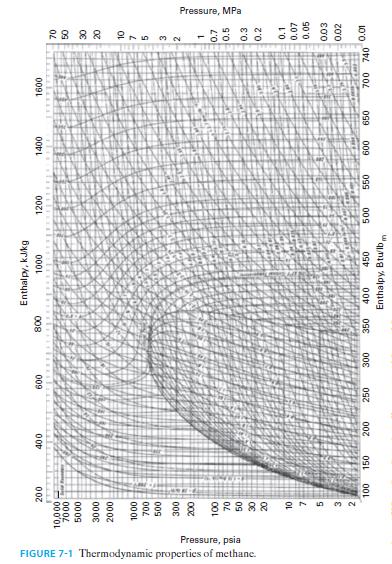
D. Comment of the results, recognizing that water is highly polar and methane is non-polar.
E. Choose your own compound that is more polar than methane, but less polar than water, and repeat parts A and B for this compound.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





