A flow of 0.02 kmol/s methane, CH 4 , and 200% theoretical air, both at reference conditions,
Question:
A flow of 0.02 kmol/s methane, CH4, and 200% theoretical air, both at reference conditions, are compressed separately to P3 = P4 = 2 MPa, then mixed and then burned in a steady-flow setup (like a gas turbine). After combustion, state 6, heat transfer goes out, so the exhaust, state 7, is at 600 K.
a. Find T3, T4, and T5.
b. Find the total rate of irreversibility from inlet to state 5.
c. Find the rate of heat transfer minus the work terms ( ˙Q − ˙W 1 − ˙W 2).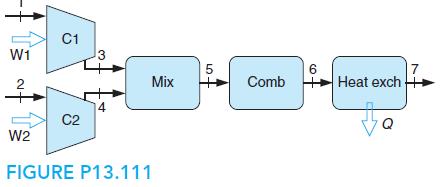
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





