Consider a gasifier that receives 4 kmol CO, 3 kmol H 2 , and 3.76 kmol N
Question:
Consider a gasifier that receives 4 kmol CO, 3 kmol H2, and 3.76 kmol N2 and brings the mixture to equilibrium at 900 K, 1 MPa, with the following reaction:
2 CO+ 2H2 ⇔ CH4 + CO2
which is the sum of Eqs. 14.32 and 14.33. If the equilibrium constant is K = 2.679, find the exit flow composition.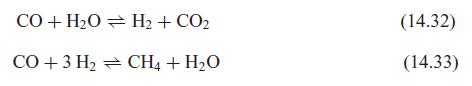
Transcribed Image Text:
CO + H20 = H2 + CO2 (14.32) CO + 3 H2 = CH4 + H20 (14.33)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
To find the exit flow composition we need to calculate the extent of reaction and then use the equil...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Consider an initial mixture of N2 and H2 gases that can be represented as follows. The gases react to form ammonia gas (NH3) as represented by the following concentration profile. a. Label each plot...
-
A mixture of 1 kmol of CO and 2 kmol of O2 is heated to 2000 K at a pressure of 2 atm. Determine the equilibrium composition of (a) O2, (b) CO2 (in kmols) assuming the mixture consists of CO2, CO and...
-
The equilibrium reaction with methane as CH4 C + 2H2 has ln K = -0.3362 at 1440 R and ln K = -4.607 at 1080 R. By noting the relation of K to temperature, show how you would...
-
A group of fraud examiners is coordinating an investigation at a local law firm. Several lawyers at the firm are suspected of overbilling clients, possibly creating fake client accounts, and then...
-
Required Prepare General Journal entries to record the following transactions of Billington Company. 2015 Jan. 10, Accepted a $3,000, 60-day, 6% note dated this day in granting a time extension on...
-
Give quantitative directions for preparing a 0.0520-m aqueous solution of sodium carbonate. Assuming an ideal vant Hoff factor, what is the expected freezing point of this solution?
-
The discipline of neuroscience makes use of network theory to identify the structures relating to functionality of the brain. Consider the paper by Vrtes et al. [469]. You do not need to study all...
-
Woods Corporation expects to incur indirect overhead costs of $60,000 per month and direct manufacturing costs of $11 per unit. The expected production activity for the first four months of 2012 is...
-
def average_grade(adict): """ Returns the average grade among all students. The dictionary adict has netids for keys and numbers 0-100 for values. These represent the grades that the students got on...
-
The Konishi Electronics Company manufactures computer microchips. A particular job that has been under analysis as part of a quality-improvement program was the subject of a time study. The time...
-
A coal gasifier produces a mixture of 1 CO and 2H 2 that is fed to a catalytic converter to produce methane. This is the methanation reaction in Eq. 14.33 with an equilibrium constant at 600 K of K =...
-
Repeat the previous problem, assuming the argon constitutes 1% of a gas mixture where we neglect any reactions of other gases and find the pressure that will give a mole concentration of A r + of...
-
Find a standard form of the given equation. y = 2x + 3
-
Amazon.com, which started as the biggest online bookstore, has become a household name by expanding rapidly in the retail market. It offers millions of movies, games, music, electronics, and other...
-
Detailed analysis of Roots Canada through porters five generic forces strategy ( explain each one of them) and give suggestions on what the company should do?
-
What role does social inequality play in shaping access to resources, opportunities, and life chances for individuals and groups within society?
-
Output the file content with line number to another text file So if an input file named "fred.txt" contains: Hello, I am Fred. I am enrolled in ICT167. Bye And the user enters "fred.txt" and "fred...
-
there's a logical error with this code. hope you can fix this. hope you can make this code more logical and add some explanation. Output - FoodOrder (run) x run: Please enter your name: a Welcome a!...
-
A police car's radar gun emits microwaves with a frequency of f1 = 36.0 GHz. The beam reflects from a speeding car, which is moving away at 43.0 m/s with respect to the police car. The frequency of...
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
When aniline is treated with fuming sulfuric acid, an electrophilic aromatic substitution reaction takes place at the meta position instead of the para position, despite the fact that the amino group...
-
Para-Nitroaniline is an order of magnitude less basic than meta-nitroaniline. (a) Explain the observed difference in basicity. (b) Would you expect the basicity of ortho-nitroaniline to be closer in...
-
Methadone is a powerful analgesic that is used to suppress withdrawal symptoms in the rehabilitation of heroin addicts. Identify the major product that is obtained when methadone is subjected to a...
-
Perform the first three steps that you would do to sort the array using mergesort. When splitting up the array or a subarray, if the (sub)array size is odd, make the left half of the subarray the...
-
*Javascript Requirements: You are not creating a visualization of Conway's Game of Life. You are simply writing a function named stepBoard that takes a 2D array of booleans and returns a 2D array of...
-
We will invest 30% of your initial investment in Stock A, 30% in Stock B and 40% in Stock C. 1. What is expected rate of return for Stock A? (0.1 point) 2. What is variance of Stock A? (0.2 point) 3....

Study smarter with the SolutionInn App


