(A) Consider a galvanic cell based on the following half-reactions. Assuming the cell operates under standard conditions...
Question:
(A) Consider a galvanic cell based on the following half-reactions. Assuming the cell operates under standard conditions at 25 °C, what is the spontaneous cell reaction? Under what nonstandard conditions is the spontaneous formation of [PtCl6]2– favored? Do you think that, in practical terms, a significant amount of PtCl62– can be obtained by altering the concentrations in a galvanic cell?
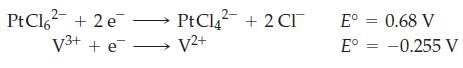
(B) Because metallic titanium exhibits excellent corrosion resistance, it is often desirable to coat iron objects with a thin coating of titanium metal. One approach involves production of Ti(s) from electrolysis of molten mixtures of NaCl and TiCl2. The production of Ti(s) involves the disproportionation of Ti2+ to Ti3+ and Ti. Write a balanced chemical equation for the disproportionation reaction and use the following half-reactions to decide whether the disproportionation reaction is spontaneous under standard conditions. Ti2+ + 2e– → Ti, E° = –1.630 V; Ti3+ + e– → Ti2+, E° = –0.369 V.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





