(A) Nitric acid, HNO 3 is produced from ammonia and oxygen by the consecutive reactions How many...
Question:
(A) Nitric acid, HNO3 is produced from ammonia and oxygen by the consecutive reactions
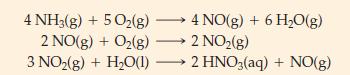
How many grams of nitric acid can be obtained from 1.00 kg NH3(g), if NO(g) in the third reaction is not recycled?
(B) Nitrogen gas is used to inflate automobile air bags. The gas is produced by detonation of a pellet containing NaN3, KNO3, and SiO2 (all solids). When detonated, NaN3 decomposes into N2(g) and Na(l). The Na(l) reacts almost instantly with KNO3 producing more N2(g) as well as K2O and Na2O. The K2O and Na2O are each converted independently into harmless salts, Na2SiO3 and K2SiO3, when they react with SiO2. Balanced chemical equations for the reactions are given below.
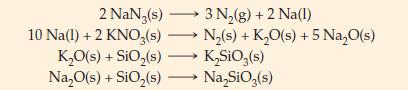
What are the minimum masses of KNO3 and SiO2 that must be mixed with 95 g NaN3 to ensure that no Na, Na2O, or K2O remains after the mixture is detonated?
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





