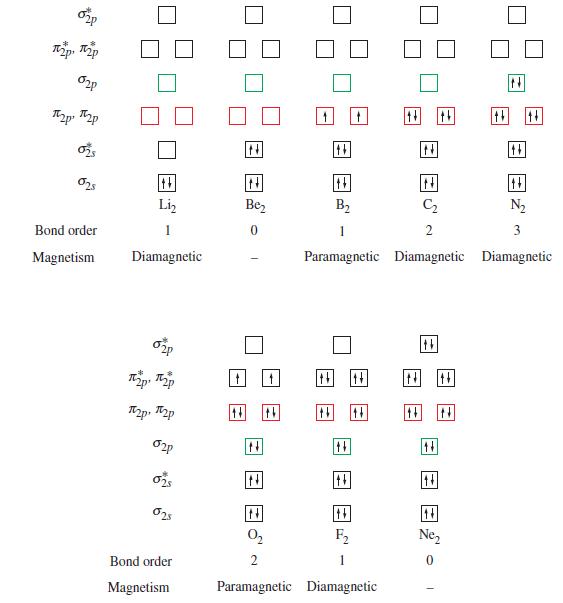
(A) Refer to Figure 11-26. Write a molecular orbital occupancy diagram, determine the bond order, and write...
Question:
(A) Refer to Figure 11-26. Write a molecular orbital occupancy diagram, determine the bond order, and write the electronic configurations of
(a) N2+;
(b) Ne2+;
(c) C22-.
(B) The bond lengths for O2+, O2, O2- and O22- are 112, 121, 128, and 149 pm, respectively. Are these bond lengths consistent with the bond order determined from the molecular orbital occupancy diagram? Explain.
Figure 11-26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





