A sample of NH 4 HS(s) is placed in a 2.58 L flask containing 0.100 mol NH
Question:
A sample of NH4HS(s) is placed in a 2.58 L flask containing 0.100 mol NH3(g). What will be the total gas pressure when equilibrium is established at 25 °C?
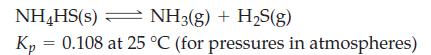
Transcribed Image Text:
NH4HS(s) NH3(g) + H₂S(g) Kp 0.108 at 25 °C (for pressures in atmospheres) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To determine the total gas pressure when equilibrium is established well first ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Sodium hydrogen carbonate (baking soda) decomposes at elevated temperatures and is one of the sources of CO 2 (g) when this compound is used in baking. What is the partial pressure of CO 2 (g)...
-
Weatherford International The oilfield services industry includes thousands of companies large and small that provide drilling, seismic testing, transportation, and a wide range of other services to...
-
(A) What will be the total gas pressure, in bar, if 12.5 g Ne is added to the mixture of gases described in Example 6-11 and the temperature is then raised to 55 C? (B) 2.0 L of O 2 (g) and 8.0 L of...
-
A project is proposed to design a database for shops selling dairy products. Each shop has a unique ID, name, address and owner. Different shops could be owned by the same owner. Each shop sells...
-
Charter Enterprises currently has $1 million in total assets and is totally equity financed. It is contemplating a change in its capital structure. Compute the amount of debt and equity that would be...
-
Timothy retains Cynthia, an attorney, to bring a lawsuit upon a valid claim against Vincent. Cynthia fails to make herself aware of recently enacted legislation that shortens the statute of...
-
Monroe Bradstad borrowed \($100,000\) from his aunt, Jeanne Garland, to purchase farmland. Both parties subsequently signed a promissory note stipulating that interest would be accrued prior to or on...
-
Thome and Crede, CPAs, are preparing their service revenue (sales) budget for the coming year (2017). The practice is divided into three departments: auditing, tax, and consulting. Billable hours for...
-
Explain the relationship and the difference between online analytical processing systems and customer relationship management systems within a business intelligence program.?
-
The following reaction is used in some self-contained breathing devices as a source of O 2 (g). Suppose that a sample of CO 2 (g) is added to an evacuated flask containing KO 2 (s) and equilibrium is...
-
One important reaction in the citric acid cycle is Write the equilibrium constant expression for the above reaction. Given that the concentrations of [citrate(aq)] = 0.00128 M, [aconitate(aq)] = 4.0...
-
In an audit report, what three opinions are expressed by the auditor?
-
Appliance Company currently has three product lines with the following information: Basic Ultra Superior Sales $2,000,000$1,350,000$650,000 Variable Expenses $1,200,000$600,000$450,000 Direct Fixed...
-
In what ways can we refine cost allocations to make them more useful for decision making?
-
Given the following financial data, calculate the following (show all calculations): 1. Gross profit/margin 2. Operating income 3. Earnings before taxes 4. Income taxes 5. Net income Tax rate Cost of...
-
Could you provide elucidations on ethnocentrism, cultural relativism, and cultural imperialism, along with illustrative examples to elucidate each concept?
-
exc!-2 Jobe date of 201 ha. Find the minimum service Jate at a printer buffer cet printer to the expected queule eize then 3 and the probability of then 3 not more a arrive new job requaring mode...
-
Describe the homeless narrator. What does Jackson Jackson mean when he says that he's been "disappearing" slowly but surely, "piece by piece"? Compare those lines with the closing lines (breathtaking...
-
Baxter, Inc., owns 90 percent of Wisconsin, Inc., and 20 percent of Cleveland Company. Wisconsin, in turn, holds 60 percent of Clevelands outstanding stock. No excess amortization resulted from these...
-
As a preliminary to requesting budget estimates of sales, costs, and expenses for the fiscal year beginning January 1, 2011, the following tentative trial balance as of December 31, 2010, is prepared...
-
How often should standards be revised?
-
How are standards used in budgetary performance evaluation?
-
Write SQL queries to solve the following problems. We will be using the "university" database. All queries should involve only the instructor table. After you are done, save your work as .sql file...
-
In Android language(Java language): Develop an application for the Shopping Cart. The application should store the Buyer Name. Order Amount and Products in the database (one table). Each Order is...
-
Could you investigate what would be the proper use of sunscreen? For example: how often should we apply sunscreen when we are at the beach? How much sunscreen should we apply? Another question: what...

Study smarter with the SolutionInn App


