Question: A solution is prepared having these initial concentrations: [Fe 3+ ] = [Hg 2 2+ ] = 0.5000 M; [Fe 2+ ] = [Hg 2+
A solution is prepared having these initial concentrations: [Fe3+] = [Hg22+] = 0.5000 M; [Fe2+] = [Hg2+] = 0.03000 M. The following reaction occurs among the ions at 25 °C.
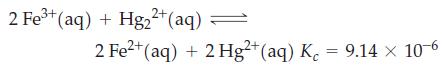
What will be the ion concentrations at equilibrium?
2 Fe+ (aq) + Hg2+ (aq) 2 Fe+ (aq) + 2 Hg2+ (aq) K = 9.14 x 10-6
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Setting up the problem The balanced chemical equation for the reaction is 2 Fe3 aq Hg22 aq 2 Fe2 aq ... View full answer

Get step-by-step solutions from verified subject matter experts


