Question: A solution prepared by dissolving 1.12 mol NH 4 Cl in 150.0 g H 2 O is brought to a temperature of 30 C. Use
A solution prepared by dissolving 1.12 mol NH4Cl in 150.0 g H2O is brought to a temperature of 30 °C. Use Figure 14-10 to determine whether the solution is unsaturated or whether excess solute will crystallize.
Figure 14-10
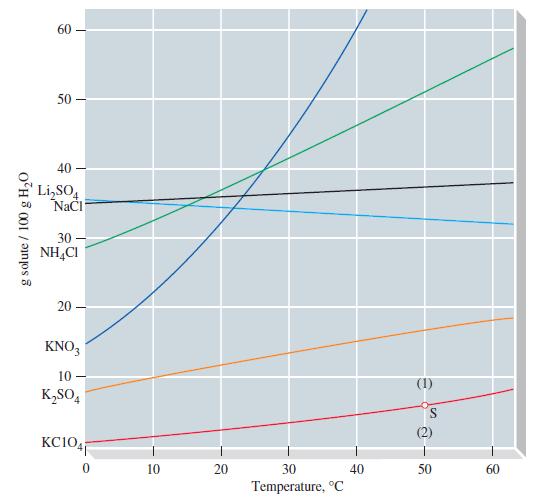
g solute/ 100 g HO 60 50- 40 LiSO4 NaCl 30 NH4Cl 20 KNO3 10- KSO4 KC1041 10 20 30 Temperature, C 40 S 50 60
Step by Step Solution
3.55 Rating (166 Votes )
There are 3 Steps involved in it
According to Figure 1410 the solubility of NH4Cl in water at 30 C ... View full answer

Get step-by-step solutions from verified subject matter experts


