Question:
(A) Write chemical equations for the reactions that occur when NaCN is dissolved in water and when Al(NO3)3 is dissolved in water. Then, use data from Appendix D to explain why a precipitate of Al(OH)3 forms when equal volumes of 1.0 M aqueous solutions of NaCN and Al(NO3)3 are mixed.
(B) The compound BeCl2 · 4 H2O cannot be dehydrated by heating and it dissolves in water to give an acidic solution. Conversely, CaCl2 · 6 H2O can be dehydrated by heating and it dissolves in water to give a solution with neutral pH. Explain these observations and write chemical equations for the reactions that occur, if any, when the salts are heated and when they are dissolved in water.
Transcribed Image Text:
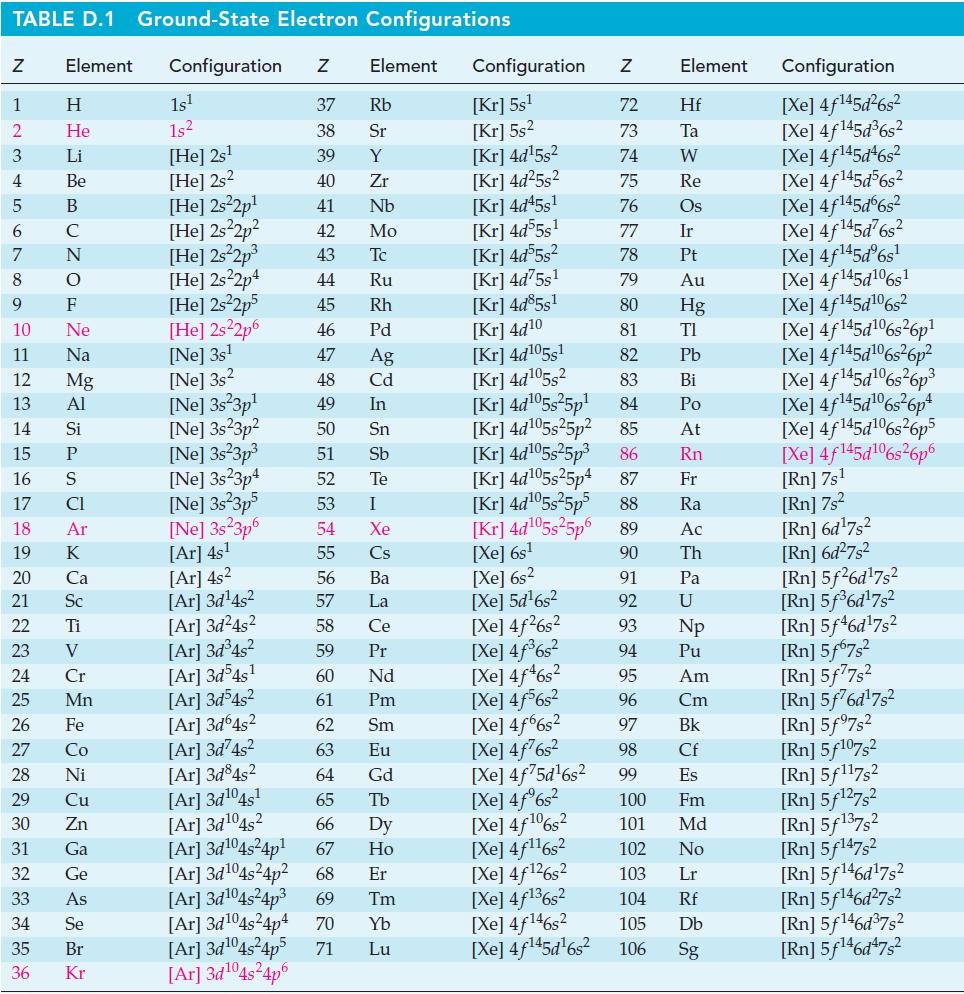
TABLE D.1 Ground-State Electron Configurations
Element Configuration Z
Z
1
2
3
4
5
6
7
8
9
HIG&LUZONSUZ SE> 0 ≤ 2 3 2 3 5 3 3 2 2 5 2
He
10
11
12
13
14
15
16
17
18
19
20
21 Sc
22
23
24
25 Mn
Mg
26
27
28 Ni
29
30 Zn
31
32
33
34
35
36
Ga
Ge
1s¹
1s²
[He] 2s¹
[He] 2s2
[He] 2s²2p¹
[He] 2s²2p²
[He] 2s²2p³
[He] 2s22p4
[He] 2s²2p5
[He] 2s²2p6
[Ne] 3s¹
[Ne] 3s2
[Ne] 3s 3p¹
[Ne] 3s23p²
[Ne] 3s²3p³
[Ne] 3s23p4
[Ne] 3s²3p5
[Ne] 3s23p6
[Ar] 4s¹
[Ar] 4s²
[Ar] 3d¹4s²
[Ar] 3d²4s²
[Ar] 3d³4s²
[Ar]3d54s¹
[Ar] 3d³4s²
[Ar] 3d64s²
[Ar] 3d²4s²
Element
37 Rb
38
Sr
39
Y
40
Zr
41 Nb
42
Mo
43
Tc
44
Ru
45 Rh
46
Pd
47
Ag
48
Cd
49
In
50
Sn
51 Sb
52
Te
53
54
55
56
57
58
59
60
61
62
63
64
I
Xe
Cs
Ba
La
Ce
Pr
Nd
Pm
Sm
Eu
Gd
[Ar]3d845²
[Ar] 3d¹04s¹
67
[Ar] 3d¹04s2
[Ar] 3d¹04s²4p¹
[Ar]3d¹04s²4p²
[Ar]3d¹04s²4p³ 69 Tm
68
Er
[Ar] 3d¹04s²4p4 70 Yb
[Ar] 3d¹04s²4p5 71
Lu
[Ar]3d¹04s²4p6
65 Tb
66
Dy
Ho
Configuration Z
[Kr] 5s¹
[Kr] 5s²
[Kr] 4d¹5s²
[Kr] 4d²5s²
[Kr] 4d45s¹
[Kr] 4d55s¹
[Kr] 4d55s²
[Kr] 4d75s¹
[Kr] 4d85s1
[Kr] 4d10
[Kr] 4d105s1
[Kr] 4d¹05s²
[Kr] 4d¹05s²5p¹
[Kr] 4d¹05s25p²
[kr] 4d¹05s²5p³
[kr] 4d¹05s25p4
[Xe] 6s²
[Xe] 5d¹6s²
[Xe] 4f²6s²
[Xe] 4f³6s²
[Xe] 4f46s2
[Xe] 4f6s2
[Xe] 4f6s2
[Xe] 4f76s2
[Xe] 4f75d¹6s²
[Xe] 4f%s2
[Xe] 4f106s2
[Xe] 4f¹¹6s²
NRNKERKR
[Xe] 4f126s2
[Xe] 4f136s2
[Xe] 4f146s2
[Xe] 4f¹45d¹6s²
72
Hf
73 Ta
W
74
75
Re
76 Os
77
Element
78
79
80
81
82
83
84
85
86
87 Fr
[Kr] 4d¹05s²5p5 88 Ra
[Kr] 4d¹05s²5p6
89
Ac
[Xe] 6s¹
90
Th
91
92
93
94
95
96
97
98
99
Ir
Pt
Au
Hg
TI
Pb
Bi
Po
At
Rn
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
100
Fm
101 Md
102 No
103 Lr
104 Rf
105
Db
106
Sg
Configuration
[Xe] 4f¹45d²6s²
[Xe] 4f145d³6s²
[Xe] 4f145d46s2
[Xe] 4f145d56s2
[Xe] 4f145d6s2
[Xe] 4f¹45d²6s²
[Xe] 4f¹45dº6s¹
[Xe] 4f145d106s1
[Xe] 4f145d106s2
[Xe] 4f145d6s26p*
[Xe] 4f145d106s36p?
[Xe] 4f145d16s?6p3
[Xe] 4f145d6s®6p*
[Xe] 4f145d16s26p5
[Xe] 4f145d106s26p6
[Rn] 7s¹
[Rn] 7s²
[Rn] 6d¹7s²
[Rn] 6d²7s²
[Rn] 5f26d¹7s²
[Rn] 5f³6d¹7s²
[Rn] 5f46d¹7s2
[Rn] 5f67s²
[Rn] 5f77s²
[Rn] 5f76d¹7s²
[Rn] 5f97s2
[Rn] 5f107,2
[Rn] 5f117s2
[Rn] 5f¹27s²
[Rn] 5f137,2
[Rn] 5f147s2
Rn] 5f¹46d¹7s²
[Rn] 5f¹46d²7s²
[Rn] 5f¹46d³7s2
[Rn] 5f¹46d47s²







