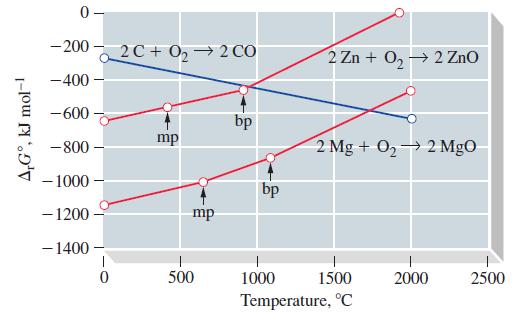
According to Figure 23-8, r G decreases with temperature for the reaction 2 C(s) + O
Question:
According to Figure 23-8, ΔrG° decreases with temperature for the reaction 2 C(s) + O2(g) → 2 CO(g). How would you expect ΔrG° to vary with temperature for the following reactions?
Figure 23-8
Transcribed Image Text:
(a) C(s) + O₂(g) (b) 2 CO(g) + O₂(g) CO₂(g) 2 CO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a Cs O2g CO2g The reaction Cs O2g CO2g is more exothermic than the reaction 2 Cs O2g 2 COg because t...View the full answer

Answered By

Deborah Joseph
My experience has a tutor has helped me with learning and relearning. You learn everyday actually and there are changes that are made to the curriculum every time so being a tutor has helped in keeping me updated about the present curriculum and all.
I have also been able to help over 100 students achieve better grades particularly in the categories of Math and Biology both in their internal examinations and external examinations.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Sports Folks Ltd is a UK resident company. The company trades in sports goods and wears. All products are taxable unless stated otherwise. Sports Folks Ltd buys goods from local manufacturers for...
-
How would you expect the extent of overlap of the bonding atomic orbitals to vary in the series IF, ICl, IBr, and I2? Explain your answer.
-
The cylinder in the figure has a radius of 2 ft and rolls without slipping on the horizontal surface. The bar AB is attached to the periphery of the cylinder by a pin at point A. The other end of the...
-
Es Diamond Shop is computing its inventory and cost of goods sold for November 2012. At the beginning of the month, these items were in stock: During the month, the shop purchased four type A rings...
-
Brendas Bar and Grill has total assets of $15 million of which $5 million are current assets. Cash makes up 10 percent of the current assets and accounts receivable makes up another 40 percent of...
-
Jason Cannon purchased a defective tractor for which Cannon brought numerous causes of action against Bodensteiner Implement Company (Bodensteiner). One of the claims was breach of express...
-
Cusick Department Store is located near the Village Shopping Mall. At the end of the company??s calendar year on December 31, 2012, the following accounts appeared in two of its trial balances....
-
e3z (i) Find all the poles of the function f (z) = and plot them on 22(22 +22+2) (ii) an Argand diagram. Hence evaluate the integral of (2) dz, writing your solutions in the form a + jb where a and b...
-
Suggest a series of reactions, using common chemicals, by which each of the following syntheses can be performed. (a) Cu(OH) 2 (s) from CuO(s). (b) CrCl 3 (aq) from (NH 4 ) 2 Cr 2 O 7 (s).
-
Suggest a series of reactions, using common chemicals, by which each of the following syntheses can be performed. (a) Fe(OH) 3 (s) from FeS(s). (b) BaCrO 4 (s) from BaCO 3 (s) and K 2 Cr 2 O 7 (aq).
-
What was TELUS' return on assets for the years ended December 31, 2011 and 2010? Interpret the ratios you calculated.
-
Suppose that you have ten observations that have a mean of 7, a median of 6, and a standard deviation of 3. If you subtract 2 from the value of each observation, what are the new values of the a....
-
Lisa Cooper is an employee of a company that manufactures tear-proof tracksuits. Lisa is paid $60 per hour, and is the only person on staff who is trained to operate a piece of technology that is...
-
The CPI was 51 in 1800 and 25 in 1900. What was the annual percentage rate of change during this 100-year period?
-
Suppose you and your teammates have been tasked by a Japanese global manufacturing corporation to organize the recruitment of employees for a major vehicle manufacturing and assembly plant in the...
-
On a chilly Sunday morning, Shaz Granger bought 30 dozen doughnuts for $75, prepared 10 litres of hot coffee and went to a farm auction. There, she sold all the coffee and all but six dozen of the...
-
What are the general criteria for eligibility for the childand dependent-care credit?
-
After graduating from college and working a few years at a small technology firm. Preet scored a high-level job in the logistics department at Amex Corporation. Amex sells high-quality electronic...
-
Azen Corporation issued $400,000, 7%, 20-year bonds on January 1, 2012, for $360,727. This price resulted in an effective-interest rate of 8% on the bonds. Interest is payable annually on January 1....
-
Perez Company issued $380,000, 7%, 10-year bonds on January 1, 2012, for $407,968. This price resulted in an effective-interest rate of 6% on the bonds. Interest is payable annually on January 1....
-
Kitov Co. receives $280,000 when it issues a $280,000, 6%, mortgage note payable to finance the construction of a building at December 31, 2012. The terms provide for semiannual installment payments...
-
The government of Canada wants to make housing more affordable. It has asked you for advice on imposing a policy in the housing market. You first look at actual data to understand how this market...
-
Building Spreadsheet Models Practice The economic principles of supply and demand are important for determining the market price for a particular product. The price for the product can also be used...
-
Analyze the graph and its corresponding phylogenetic tree below. During which period was the rate of speciation highest? Number of lineages 8- 4 A B C 2- Relative time since root node

Study smarter with the SolutionInn App


