Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard
Question:
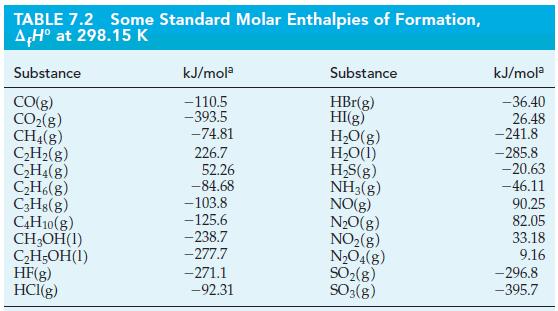
Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard enthalpy of combustion for formic acid (ΔrH° = -255 kJ/mol) to calculate the standard enthalpy of formation for formic acid.
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, AH° at 298.15 K Substance CO(g) CO₂(g) CH4(8) C₂H₂(g) C₂H4(8) C₂H6(g) C3H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The standard enthalpy change for the combustion of formic acid can be represented as follows HCOOHI ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpy of combustion of ethene gas [C2H4(g)] is 1411.1 kJ/ mol at 298 K. Given the following enthalpies of formation, calculate Hof for C2H4(g). CO2(g) 393.5 kJ/ mol H2O(l) 285.8 kJ/...
-
The standard enthalpy of combustion of cyclopropane is 2091 kJ mol 1 at 25C. From this information and enthalpy of formation data for CO 2 (g) and H 2 O(g), calculate the enthalpy of formation of...
-
The standard enthalpy of combustion of solid urea (CO (NH2)2) is -632 kl mol-1 at 298 K and its standard molar entropy is 104.60 J K-1 mol-1, Calculate the standard Gibbs energy of formation of urea...
-
Consider the following types of images: i. Real, inverted and highly diminished image. ii. Real, inverted and enlarged image. iii. Virtual, erect and enlarged image. iv. Virtual, erect and diminished...
-
Managerial accounting is more than recording, maintaining, and reporting financial results. Managerial accountants must provide managers with both financial and nonfinancial information including...
-
Calculate return on capital employed for a sole proprietor whose net profit was 90,000 and whose capital employed was 270,000.
-
For approximately 20 months, Robert E. McDonald perpetrated a scheme to solicit millions of dollars purportedly for a \($100\) million purchase by the RAI Entities and certain other related corporate...
-
Jobs, Inc. has recently started the manufacture of Tri-Robo, a three-wheeled robot that can scan a home for fires and gas leaks and then transmit this information to a mobile phone. The cost...
-
In Figure 32.14, the battery has an emf of 12.0 V, the inductance is L, and the capacitance C is 9.0 pF. The switch has been set to position a for a long time so that the capacitor is charged. The...
-
As the manager of a focus group company, you are interested in optimizing the number of participants you include in focus groups for your clients. Over the past year, you ran a field experiment,...
-
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the standard enthalpy of formation for lactic acid [CH 3 CH(OH)COOH(s)]: f H = -694.0 kJ/mol. Table 7.2 TABLE...
-
The decomposition of limestone, CaCO 3 (s), into quicklime, CaO(s), and CO 2 (g) is carried out in a gas-fired kiln. Use data from Appendix D to determine how much heat is required to decompose 1.35...
-
Go to www.ge.com and follow the link to Investor Relations, and then to Financial Reporting to see General Electric s (GE) most recent annual report. Answer the following questions about GE: 1. Look...
-
What do you believe are the strengths and challenges of the Patient Centered Medical Home? Please explain why?
-
Random Variable X has the pdf f(x)={ a) Find P(1 < X <2) (3 points): b) Find P(5
-
Assume Smiley Inc. will pay a dividend of $5 per share at the end of Year 1 after which dividends will grow at a constant rate of 3% forever. Smiley has a cost of equity of 10%. If you value the firm...
-
A 6-month note is issued on October 1. If no previous accruals have been made, how many months of interest should be accrued at December 31? Show your calculation.
-
You trade gold futures. The price of one ounce of gold is $1000. The interest rate is 0%. The annual storage cost of gold is $20 per ounce per year. There are two gold futures contracts. First...
-
ExxonMobil Corporation, the worlds second-largest company, uses the LIFO inventory method for most of its inventories. Its inventory costs are heavily dependent on the cost of oil. When the price of...
-
A stock has had returns of 8 percent, 26 percent, 14 percent, 17 percent, 31 percent, and 1 percent over the last six years. What are the arithmetic and geometric average returns for the stock?
-
Capitalization of Interest on July 31, 2010, Bismarck Company engaged Duval Tooling Company to construct a special-purpose piece of factory machinery. Construction was begun immediately and was...
-
Capitalization of Interest the following three situations involve the capitalization of interest. Situation I On January 1, 2010, Columbia, Inc. signed a fixed-price contract to have Builder...
-
Entries for Equipment Acquisitions Chopin Engineering Corporation purchased conveyor equipment with a list price of $15,000. Presented below are three independent cases related to the equipment....
-
Lonnie lanazzo, owner of Anthony's Jewelers in Tulsa, Oklahoma, marks up diamond earrings 83% on cost. The earrings cost Anthony's Jewelers $120 each. At what price should Anthony's sell the...
-
The 7 percent preferred stock of Midwest Muffler and Towing is selling for $65 per share. What is the firm's cost of preferred stock if the tax rate is 21 percent and the par value per share is $100?
-
A rocket lifts off firing two engines simultaneously. One has an upward thrust of 8 0 0 [ N ] . The other, a 4 0 0 [ N ] thrust at 3 0 to the right of the first engine. What is the magnitude and...

Study smarter with the SolutionInn App


