As discussed in Are You Wondering 11-1, the sp hybrid orbitals are algebraic combinations of the s
Question:
As discussed in Are You Wondering 11-1, the sp hybrid orbitals are algebraic combinations of the s and p orbitals. The required combinations of 2s and 2p orbitals are
![$1(sp): 14 (25) [4(2s) +(2pz)] 1 42(sp) = [4 (25) 4 (2p)] -](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/0/2/0/03365543f41995851700020031923.jpg)
(a) By combining the appropriate functions given in Table 8.2, construct a polar plot in the manner of Figure 8-24 for each of the above functions in the xz plane. In a polar plot, the value of r/a0 is set at a fixed value. Describe the shapes and phases of the different portions of the hybrid orbitals, and compare them with those shown in Figure 11-12.
(b) Convince yourself that the combinations employing the 2px or 2py orbital also give similar hybrid orbitals but pointing in different directions.
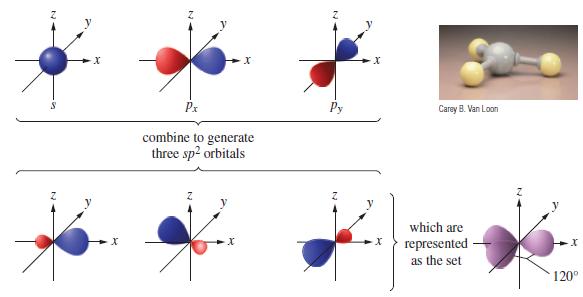
(c) The combinations for the sp2 hybrids in the xy plane are
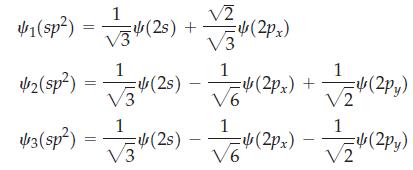
By constructing polar plots (in the xy plane), show that these functions correspond to the sp2 hybrids depicted in Figure 11-10.
Table 8.2
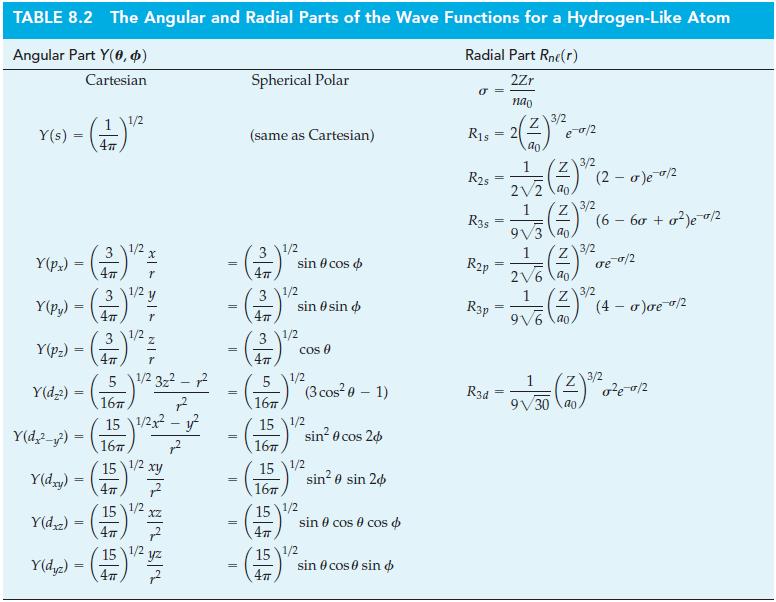
Figure 8-24
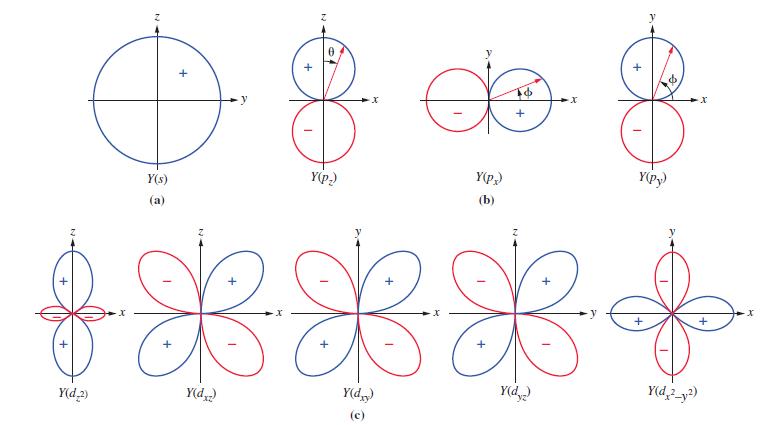
Figure 11-12
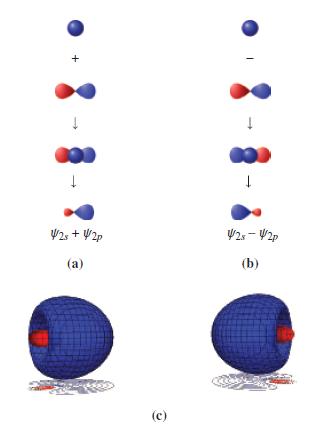
Figure 11-10
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




