At 298 K, the f G values for Li 2 O(s) and Li 2 O 2
Question:
At 298 K, the ΔfG° values for Li2O(s) and Li2O2(s) suggest that Li2O2(s) is thermodynamically more stable than Li2O(s). At 1000 K, however, the situation is reversed. The standard Gibbs energies of formation, ΔfG°, for Li2O(s) and Li2O2(s) are –466.40 kJ mol‾1 and –419.02 kJ mol‾1, respectively, at 1000 K. Calculate the equilibrium constant for the following reaction at 1000 K and the equilibrium partial pressure of O2(g) above a sample of Li2O2(s) at 1000 K.
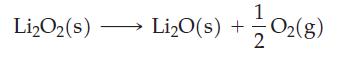
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





