At 65 C, the half-life for the first-order decomposition of N 2 O 5 (g) is 2.38
Question:
At 65 °C, the half-life for the first-order decomposition of N2O5(g) is 2.38 min.
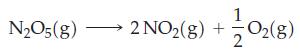
If 1.00 g of N2O5 is introduced into an evacuated 15 L flask at 65 °C,
(a) What is the initial partial pressure, in mmHg, of N2O5(g)?
(b) What is the partial pressure, in mmHg, of N2O5(g) after 2.38 min?
(c) What is the total gas pressure, in mmHg, after 2.38 min?
Transcribed Image Text:
N₂O5 (g) 2 NO2(g) + O₂(g) 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a To calculate the initial partial pressure of N2O5g we can use the ideal gas law PV nRT where P is ...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 would be 0.50 atm at 523 K. However, PCl5 decomposes to gaseous PCl3 and Cl2, and the actual pressure...
-
If 10.00 g of water are introduced into an evacuated flask of volume 2.500 L at 65C, calculate the mass of water vaporized. (Assume that the volume of the remaining liquid water is negligible; the...
-
(1) Choose all of the following statements that are correct about the time evolution of a general wave function: (I) The time evolution of a general wave function is governed by the Hamiltonian...
-
Largest Company acquired Large Company on January 1. As part of the acquisition, $10,000 in goodwill was recognized; this goodwill was assigned to Largest's Production reporting unit. During the...
-
The stockholders equity section of YUM! Brands, Inc., the operator of Pizza Hut, KFC, and Taco Bell restaurants, for two recent comparative dates was as follows: 1. What is the other comprehensive...
-
Sumitomo Cable manufactures various types of aluminum and copper cables which it sells directly to retail outlets through its distribution channels. The manufacturing process for producing cables...
-
On January 1, 2014, Kessler Inc. had these stockholders equity balances. Common Stock , $1 par (2,000,000 shares authorized, 600,000 shares issued and outstanding).................$ 600,000 Paid-in...
-
Find the perimeter of the figure shown. Express the perimeter using the same unit of measure that appears on the given sides. 13 yd Perimeter = 5 yd 5 yd 13 yd 18 yd 18 yd
-
An alternative mechanism of the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) follows. Show that this mechanism is consistent with the rate law, equation (20.24). Eq. 20.24 Fast: Slow: Overall: NO(g) + O(g)...
-
(A) In a proposed two-step mechanism for the reaction CO(g) + NO 2 (g) CO 2 (g) + NO(g), the second, fast step is NO 3 (g) + CO(g) NO 2 (g) + CO 2 (g). What must be the slow step? What would you...
-
In Exercises determine the degree of the Maclaurin polynomial required for the error in the approximation of the function at the indicated value of to be less than 0.001. cos (0.1)
-
5. What is the difference between the usage of the set difference operation in an except/minus statement and the left outer join command? 6. How do I return a result set in which the selected key is...
-
Consider a 5 stage pipeline with stages taking 2, 1, 1.5, 2, 1 units of time. What is the speed up and throughput of the pipeline for executing 500 tasks with stage buffer time of 1 unit and 1000...
-
Cost of Goods Sold Pietro Frozen Foods, Inc., produces frozen pizzas. For next year, Pietro predicts that 50,500 units will be produced, with the following total costs: Direct materials ? Direct...
-
What are two determinants at each of the three hierarchical levelsmicro, mezzo, and macrothat exert influence on an individual's engagement in physical activity? Following that, can you elaborate on...
-
An inventor wants to produce samples of a new cell phone accessory. A manufacturer will produce the samples for an upfront cost of $630 plus a per unit cost of $29 . The inventor's costs must be...
-
An accounting intern for a local CPA firm was reviewing the financial statements of a client in the electronics industry. The intern noticed that the client used the FIFO method of determining ending...
-
Frontland Advertising creates, plans, and handles advertising campaigns in a three-state area. Recently, Frontland had to replace an inexperienced office worker in charge of bookkeeping because of...
-
Glendo Industries' balance sheet at December 31, 2010, is presented below and on the next page. Additional information accumulated for the budgeting process is as follows.Budgeted data for the year...
-
Suppan Farm Supply Company manufactures and sells a fertilizer called Basic II. The following data are available for preparing budgets for Basic II for the first 2 quarters of 2010. 1. Sales: Quarter...
-
Durham Inc. is preparing its annual budgets for the year ending December 31, 2010. Accounting assistants furnish the following data. An accounting assistant has prepared the detailed manufacturing...
-
Regulations for Canadian financial institutions require that mortgage rates be quoted with Blank______.
-
Identify the last 2 stages of Erik Erikson's stages of development and give your view on whether you believe that adults go through these stage. Why are these stages important in the context of adult...
-
For a stated positive interest rate and multiple (more than one) compounding periods per year, the EAR is always Blank______ the APR.

Study smarter with the SolutionInn App


